��Ŀ����
7��������ѧ���ش��������⣺��1���л����������֪ʶ�����ʹ��еĻ�����
��
 ��ϵͳ����Ϊ��2��4-����-3-�һ����飮
��ϵͳ����Ϊ��2��4-����-3-�һ����飮��3-��-2-�����Ľṹ��ʽ��CH3CH��OH��CH��CH3��2��
���Ҷ����׳Ʋ��ᣮ
��2�������Ŷ��л���������������ã���Ҳ���ܵ��������ŵ�Ӱ�죮
�ٱȽϷе�
 ��
�� ���������������=������ͬ��
���������������=������ͬ���ڱȽ����ԣ�
 ��
�� ����ʾ����ȷ��봼�����ԣ�
����ʾ����ȷ��봼�����ԣ���3���ϳ��л����Ѿ��������������еõ��㷺��Ӧ�ã�
����д���ױ��ϳ�TNT�ķ�Ӧ����ʽ��
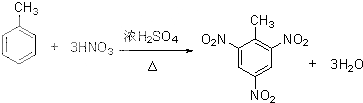 ��
������д�����ᣨ
 ���ڴ��������£��ϳɾ����ᣨPLA���ķ�Ӧ����ʽ��
���ڴ��������£��ϳɾ����ᣨPLA���ķ�Ӧ����ʽ�� ��
��
���� ��1���� ���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����3-��-2-�����Ľṹ��ʽΪ��CH3CH��OH��CH��CH3��2�����Ҷ����׳Ʋ��
���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����3-��-2-�����Ľṹ��ʽΪ��CH3CH��OH��CH��CH3��2�����Ҷ����׳Ʋ��
��2����̼ԭ����Խ�࣬�ǻ���Խ�࣬�е�Խ�ߣ�
��ȩ�������ԣ�����Ϊ��ǿ���ᣬ���Ա�̼��ǿ���ݴ˱Ƚϼ��ɣ�
��3���ٰ�ŨH2SO4��ŨHNO3�ͼױ���ϼ����Ʊ�TNT���DZ�������ԭ�ӱ�����ȡ�������������ױ�������ԭ���غ���ƽ��ѧ����ʽ��
������ͨ��������Ӧ���е����۷�Ӧ���ɾ����ᣮ
��� �⣺��1���� ���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����ȷ����Ϊ��2��4����-3-�һ����飬�ʴ�Ϊ��2��4-����-3-�һ����飻
���̼��Ϊ6���ֱ���2��4��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����ȷ����Ϊ��2��4����-3-�һ����飬�ʴ�Ϊ��2��4-����-3-�һ����飻
��3-��-2-�����Ľṹ��ʽΪ��CH3CH��OH��CH��CH3��2���ʴ�Ϊ��CH3CH��OH��CH��CH3��2��
���Ҷ���ͨ���Զ�ˮ�������ʽ���ڣ��׳Ʋ��ᾧ�壬�ʴ�Ϊ���
��2����̼ԭ����Խ�࣬�ǻ���Խ�࣬�е�Խ�ߣ��������к���3��C��3���ǻ��� �к���3��C��2���ǻ����ʱ������ķе����
�к���3��C��2���ǻ����ʱ������ķе���� ���ʴ�Ϊ������
���ʴ�Ϊ������
��ȩ�������ԣ������������ǿ��̼��ǿ������������ǿ�ڶԼ�����ȩ���ʴ�Ϊ������
��3���ٰ�ŨH2SO4��ŨHNO3�ͼױ���ϼ����Ʊ�TNT�Ļ�ѧ����ʽΪ��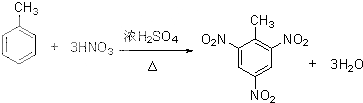 ���ʴ�Ϊ��
���ʴ�Ϊ��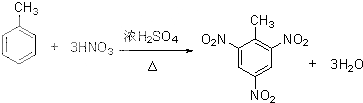 ��
��
������ͨ��������Ӧ���е����۷�Ӧ���ɾ����ᣬ��Ӧ����ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ������Ҫ��������л��������������ṹ��ʽ����д���л����۷е��Լ�����ǿ���ıȽϣ��л���ѧ��Ӧ����ʽ����д�ȣ��ۺ��Խ�ǿ����һ�����Ѷȣ�

| A�� | ���÷�Һ©�����з�Һ����ʱ���ȴ�Һ©��������ʹ�²�Һ���������������²�Һ����ȫ�����ر��������ٽ��ϲ�Һ����Ͽڵ��� | |
| B�� | �����Ӽ�ȡ�����ƹ��壬�и�ȡ�ú�ʣ����ƷŻ�ԭ�Լ�ƿ�� | |
| C�� | �ñ�Ũ�ȵ�����ζ�δ֪Ũ��NaOH��Һʱ����ʽ�ζ���������ˮϴ����δ��ͬŨ��������ϴ�������²ⶨ���ƫ�� | |
| D�� | ��Һ���ơ��к͵ζ�ʵ���У�����ƿ����ƿ������ˮϴ����ʹ�ã����ζ��ܡ���Һ��������ˮϴ���������ô�װҺ��ϴ2��3�κ�ʹ�� |
| A�� | ��ˮ | B�� | ��������Һ | C�� | NaOH��Һ | D�� | NaHCO3��Һ |
| A�� | ����BaSO4��BaSO3���� | B�� | �������� | ||
| C�� | ����BaSO4���� | D�� | ����BaSO3���� |
| Ԫ�ر�� Ԫ������ | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶 ��10-10m�� | 1.52 | 2.27 | 0.74 | 1.43 | 0.77 | 1.10 | 0.99 | 1.86 | 0.75 | 0.71 |
| ���̬ | +1 | +1 | -- | +3 | +4 | +5 | +7 | +1 | +5 | -- |
| ��ͼ�̬ | -- | -- | -2 | -- | -4 | -3 | -1 | -- | -3 | -1 |
��1������10��Ԫ�ص�ԭ���У�������ʧȥ���ӵ��Ǣڣ���д��ţ�����H2�������ϵķǽ��������Ƿ�����д�������ƣ���
��2��д��Ԫ�آݵ��⻯��ṹʽ
 ���ۺ͢��γɵĻ�����ĵ���ʽ
���ۺ͢��γɵĻ�����ĵ���ʽ ��д��Ԫ�آۺ͢��γɵļ������Ӽ������й��ۼ��Ļ�����ĵ���ʽ
��д��Ԫ�آۺ͢��γɵļ������Ӽ������й��ۼ��Ļ�����ĵ���ʽ ��
����3��ijԪ��R��ԭ�Ӱ뾶Ϊ1.02��10-10m����Ԫ�������ڱ��е�λ�õ������ڵ�VIA�壮
��4��д�������ޡ�������Ԫ���γɵĻ������У�ÿ��ԭ�Ӷ����������Ϊ8�����ȶ��ṹ��һ�����ʵķ���ʽPCl3��
��5���ԱȽϢݺ͢���Ԫ�ص�����������Ӧ��ˮ���������ǿ�����ѧʽ����HClO4��HNO3
��6��д���ܵ����������ֱ���ߺ͢������������ˮ���ﷴӦ�����ӷ���ʽOH-+Al��OH��3=AlO2-+2H2O��Al��OH��3+3H+=Al3++3H2O��
| A�� | ���ģ�� ��ʾ���л�������ϩ ��ʾ���л�������ϩ | |
| B�� | ����ʽ ���Ա�ʾ�ǻ���Ҳ���Ա�ʾ������ ���Ա�ʾ�ǻ���Ҳ���Ա�ʾ������ | |
| C�� | �л��� �ķ���ʽΪC6H8O2 �ķ���ʽΪC6H8O2 | |
| D�� | �ṹ��ʽ��CH3��2CHOH�����Ա�ʾ1-������Ҳ���Ա�ʾ2-���� |
| A�� | �������� | |
| B�� | ����Na2CO3��ͨ����ȡ�ķ������� | |
| C�� | �ȼ����ռ���Һ��֮��������ȩ���ټ���ŨH2SO4���������� | |
| D�� | ��Na��Ӧ����з��� |
 ��ȡ
��ȡ ����ϳ��������£�
����ϳ��������£�
 ��������Ӧ�Ļ�ѧ����ʽ��
��������Ӧ�Ļ�ѧ����ʽ�� ���ƣ�3��4-�������飮
���ƣ�3��4-�������飮