��Ŀ����
��ͼΪij��ʵ��װ��ʾ��ͼ��������ʵ��˵��������װ���Ѿ�ʡ�ԣ�
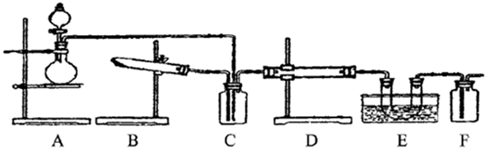
I����ͬѧ��Ϊ��D��ʢ��V2O5������ʱ������װ�ÿ����ںϳ�����X����Ԥ��E����ˮ��ȴ��U���н��й���X���֣�
��1����ʵ�ϴ�װ������ʵ��˵�����ϼ�ͬѧ���ƶϣ���ôA���������ķ�Ӧ�Ļ�ѧ����ʽ��______��Cװ����ʢ�ŵ�Һ��ҩƷ��������______��
��2����ͬѧ������Ϊ�����Aװ�ò����ת���ʣ���������Bװ�ò����ͨ��������֪A��B��װ���в�����D�е�ͨ��������ͨ���۲�______��֪��
��3����ʵ�������������ȱʧ֮�������������Ľ�����______�������ޣ����ʿɲ���
II����ͬѧ��Ϊ��D��ʢ�Ų���Ͻ����������������װ��Ҳ�����ںϳ�����Y����Ԥ��E����ˮ��ȴ��U���н��к���ɫ�������ɣ���Խ�ӽ�U�ܵײ���ɫԽdz��
��4��д����ͬѧ��Ϊ��Dװ���з�����Ӧ�Ļ�ѧ����ʽ______��
��5������A��������Ϊû�м���װ�ã�����Ҳ���A������IJ���ԭ��______��6����Ҫ������װ�������ͬѧ����Ϊ��ʵ�飬��C������������ϳ�Xʱ�������û� ��һ�£���ôC���Ĺ��ƿӦ��Ϊ______����װҩƷΪ______��

I����ͬѧ��Ϊ��D��ʢ��V2O5������ʱ������װ�ÿ����ںϳ�����X����Ԥ��E����ˮ��ȴ��U���н��й���X���֣�
��1����ʵ�ϴ�װ������ʵ��˵�����ϼ�ͬѧ���ƶϣ���ôA���������ķ�Ӧ�Ļ�ѧ����ʽ��______��Cװ����ʢ�ŵ�Һ��ҩƷ��������______��
��2����ͬѧ������Ϊ�����Aװ�ò����ת���ʣ���������Bװ�ò����ͨ��������֪A��B��װ���в�����D�е�ͨ��������ͨ���۲�______��֪��
��3����ʵ�������������ȱʧ֮�������������Ľ�����______�������ޣ����ʿɲ���
II����ͬѧ��Ϊ��D��ʢ�Ų���Ͻ����������������װ��Ҳ�����ںϳ�����Y����Ԥ��E����ˮ��ȴ��U���н��к���ɫ�������ɣ���Խ�ӽ�U�ܵײ���ɫԽdz��
��4��д����ͬѧ��Ϊ��Dװ���з�����Ӧ�Ļ�ѧ����ʽ______��
��5������A��������Ϊû�м���װ�ã�����Ҳ���A������IJ���ԭ��______��6����Ҫ������װ�������ͬѧ����Ϊ��ʵ�飬��C������������ϳ�Xʱ�������û� ��һ�£���ôC���Ĺ��ƿӦ��Ϊ______����װҩƷΪ______��
��1��V2O5������ʱ���Ƕ�������ת��Ϊ��������ʱ�ķ�Ӧ����������A����ȡ���������װ�ã��������ķ�Ӧ�Ļ�ѧ����ʽ�ǣ�Na2SO3+H2SO4��Ũ��=Na2SO4+H2O+SO2�����������������Ũ���ᣬ
�ʴ�Ϊ����Na2SO3+H2SO4��Ũ��=Na2SO4+H2O+SO2����Ũ���
��2��Cװ�õ����ã�������������ڶ�������������Ļ�ϱ������ɿ��ƶ�������������IJ������ʣ���֪���������������D�е�ͨ��������ͨ���۲�C�������ܿ����ݵIJ����������жϣ��ʴ�Ϊ��C�������ܿ����ݵIJ������ʣ�
��3��Fװ�ý���β������ʱ�����µ�������IJ���������Ӧ��EF֮������һ��������װ�ã��ʴ�Ϊ���У�Ӧ��EF֮������һ��������װ�ã�
��4������Ͻ������������ǰ����Ĵ�����ʱ�ķ�Ӧ����������Dװ���ǰ����Ĵ�������Ӧ���䷢����Ӧ�Ļ�ѧ����ʽΪ��4NH3+5O2
4NO+6H2O���ʴ�Ϊ��4NH3+5O2
4NO+6H2O������д2NO+O2=2NO2Ҳ���ԣ���
��5��������Ũ��ˮ����NaOH������ʯ�һ��ʯ�ң���ϲ����ȵķ������ư�������ԭ���ǣ���ѧƽ��NH3+H2O?NH3?H2O?NH4++OH-���ƶ�ԭ�����ʴ�Ϊ������Һ©���е�Ũ��ˮ�μ���Բ����ƿ�еĹ���NaOH������ʯ�һ��ʯ�ң��У���Ӧ������Һ��c��OH-������ˮŨ�����������������ؾ����°�ˮ�е�ƽ�⣺NH3+H2O?NH3?H2O?NH4++OH-�����ƶ���ʹNH3�ݳ���
��6�������Ǽ������壬���ü�ʯ�Ҹ��ﰱ���������������ڸ�����У��ʴ�Ϊ��U�ιܻ����ܣ���ʯ�ң�������������ɣ���
�ʴ�Ϊ����Na2SO3+H2SO4��Ũ��=Na2SO4+H2O+SO2����Ũ���
��2��Cװ�õ����ã�������������ڶ�������������Ļ�ϱ������ɿ��ƶ�������������IJ������ʣ���֪���������������D�е�ͨ��������ͨ���۲�C�������ܿ����ݵIJ����������жϣ��ʴ�Ϊ��C�������ܿ����ݵIJ������ʣ�
��3��Fװ�ý���β������ʱ�����µ�������IJ���������Ӧ��EF֮������һ��������װ�ã��ʴ�Ϊ���У�Ӧ��EF֮������һ��������װ�ã�
��4������Ͻ������������ǰ����Ĵ�����ʱ�ķ�Ӧ����������Dװ���ǰ����Ĵ�������Ӧ���䷢����Ӧ�Ļ�ѧ����ʽΪ��4NH3+5O2
| ||
| ||
��5��������Ũ��ˮ����NaOH������ʯ�һ��ʯ�ң���ϲ����ȵķ������ư�������ԭ���ǣ���ѧƽ��NH3+H2O?NH3?H2O?NH4++OH-���ƶ�ԭ�����ʴ�Ϊ������Һ©���е�Ũ��ˮ�μ���Բ����ƿ�еĹ���NaOH������ʯ�һ��ʯ�ң��У���Ӧ������Һ��c��OH-������ˮŨ�����������������ؾ����°�ˮ�е�ƽ�⣺NH3+H2O?NH3?H2O?NH4++OH-�����ƶ���ʹNH3�ݳ���
��6�������Ǽ������壬���ü�ʯ�Ҹ��ﰱ���������������ڸ�����У��ʴ�Ϊ��U�ιܻ����ܣ���ʯ�ң�������������ɣ���

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ
��15�֣�ij��ѧ��ȤС����ʵ����̽�������й����ʣ��������ͼ��ʾʵ�飬A�������巢��װ�ã�A�����õ�ʵ��ҩƷ������������ѡȡ��
�� Na2CO3�� NaOH �� Ca(OH)2�� NaHCO3�� NH4Cl
����ͼ���Ӻø��������ֽ�C���IJ�˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ơ�����ʵ����������£���˿�������ֺ���״̬��D�е�ͭƬ�����ܽ⣬�ش��������⣺
��1��A�з����Ļ�ѧ��Ӧ����ʽΪ________����ȡ�������õ��IJ���������Ҫ��_______�֣�����������װ�ã���
��2��Bװ�õ�����Ϊ____________________________�� ��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
| A��Na2CO3 | B��AgNO3 | C��H2SO4 | D��FeSO4 |
��5��������Ϊ������Ƶ�����ʵ��װ�û��������ԵIJ��㣬���䲻��֮���Լ�Ӧ��θĽ���̸̸��������_______________________________________________________��
��ͨ������˵�������ײ�ǵ�����ײ���Ƿǵ�����ײ��




 ��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�