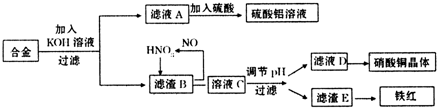
��Ŀ����
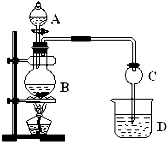
��15�֣�ij��ѧ��ȤС����ʵ����̽�������й����ʣ��������ͼ��ʾʵ�飬A�������巢��װ�ã�A�����õ�ʵ��ҩƷ������������ѡȡ��
�� Na2CO3 �� NaOH �� Ca(OH)2 �� NaHCO3 �� NH4Cl
����ͼ���Ӻø��������ֽ�C���IJ�˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ơ�����ʵ����������£���˿�������ֺ���״̬��D�е�ͭƬ�����ܽ⣬�ش��������⣺
��1��A�з����Ļ�ѧ��Ӧ����ʽΪ________����ȡ�������õ��IJ���������Ҫ��_______�֣�����������װ�ã���
��2��Bװ�õ�����Ϊ____________________________��
 ��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
A��Na2CO3 B��AgNO3 C��H2SO4 D��FeSO4
��4��ͼE�г���ͨ������������Ϊ____________________��
��5��������Ϊ������Ƶ�����ʵ��װ�û��������ԵIJ��㣬���䲻��֮���Լ�Ӧ��θĽ���̸̸��������_______________________________________________________��
��ͨ������˵�������ײ�ǵ�����ײ���Ƿǵ�����ײ��
��1��Ca(OH)2 +2NH4Cl CaCl2 +2H2O+ 2NH3����3�֣�
2��2�֣�
CaCl2 +2H2O+ 2NH3����3�֣�
2��2�֣�
��2�����ն�����̼��ˮ����������������2�֣�
��3��3Cu+8H+ + 2NO3��- = 3Cu2+ + 2NO��+ 4H2O��2�֣� B C��2�֣�
��4��ʹ�к��������ո���ȫ��2�֣�
��5��Dװ�õ�Һ�������벣����C�У�ʹ���������ѣ���Cװ����Dװ��֮������һ������װ�á���2�֣�
��������







 ij��ѧ��ȤС�����ʵ����ȡ����������������ͼװ�ý���ʵ�飬��Բ����ƿ�ڼ������Ƭ���ټ�����2mL 98%��ŨH2SO4��3mL�Ҵ���ɵĻ��Һ��ͨ����Һ©������ƿ�ڼ���2mL�����ᣬ�ձ��м��뱥��Na2CO3��Һ����ش��������⣺
ij��ѧ��ȤС�����ʵ����ȡ����������������ͼװ�ý���ʵ�飬��Բ����ƿ�ڼ������Ƭ���ټ�����2mL 98%��ŨH2SO4��3mL�Ҵ���ɵĻ��Һ��ͨ����Һ©������ƿ�ڼ���2mL�����ᣬ�ձ��м��뱥��Na2CO3��Һ����ش��������⣺