��Ŀ����
2011��3�£��ձ������˵�վ������й©����й©�ķ����������У���������һ�ֽ�������-131���ķ�����Ԫ�أ���˵�Ժ���ʳƷ�����Ƭ���Լ�����-131�������Ӱ�죬��Щ�˾�ȥ�������Ρ���Ƭ���±���ijʳ�õ��ΰ�װ���ϵIJ���˵����
| �䡡�� | ʳ�Ρ�����ء������ |
| �⺬�� | 35��15mg/kg |
| ���ط��� | �ܷ⡢�ܹ⡢���� |
| ʳ�÷��� | ���ʱ����ʳƷ��������� |
A������ؿ������Ȼ��ơ�B��ֻ�õ��۾��ܼ�������еĵ���ء� C�����»ᵼ�µ����ʧ D���õ����е���غ���ԼΪ34��84mg/kg
��2��������ڹ�ҵ�Ͽ��õ�ⷨ��ȡ����ʯī�Ͳ����Ϊ�缫����KI��ҺΪ���Һ����һ�������µ�⣬��Ӧ�ķ���ʽΪ��KI+3H2O
 KIO3+3H2��������������Ϊ______��
KIO3+3H2��������������Ϊ______����3���������⻯�������������·������·�Ӧ����ƽ��ѧ����ʽ��
______KIO3+______KI+______H2SO4��______K2SO4+______I2+______H2O
�÷�Ӧ��������Ϊ______
��4����֪��I2+2S2O32-��2I-+S4O62-��ijѧ���ⶨʳ�õ����е�ĺ������䲽��Ϊ��
a��ȷ��ȡw gʳ�Σ�����������ˮʹ����ȫ�ܽ⡡b����ϡ�����ữ������Һ����������KI��Һ��ʹKIO3��KI��Ӧ��ȫ��c���Ե���Ϊָʾ������μ������ʵ���Ũ��Ϊ2.0��10-3mol?L-1��Na2S2O3��Һ10.0mL��ǡ�÷�Ӧ��ȫ�����������е�ĺ����ǣ��Ժ�w�Ĵ���ʽ��ʾ��______mg/kg��
�⣺��1��A��ʳ���м������أ�˵�����߿ɹ��棬���ȶ����ڣ�˵������֮�䲻������Ӧ����A����
B������ֻ�ܼ���ⵥ�ʣ������ز���Ӧ����B����
C���ɡ����ʱ����ʳƷ��������Ρ�֪���»ᵼ�µ����ʧ����C��ȷ��
D���ɵ�ĺ���35��15mg/kg���õ����е���غ���ԼΪ20�� ��50��
��50�� ����34��84mg/kg����D��ȷ��
����34��84mg/kg����D��ȷ��
�ʴ�Ϊ��CD��
��2���ɵ�ⷽ��ʽ��֪���ʱKI��������������˵������Ϊ���Ե缫���ϣ�ӦΪʯī���ʴ�Ϊ��ʯī��
��3����Ӧ��KIO3��KI֮�䷢��������ԭ��Ӧ����I2����������������ԭ��֮���ʧ������Ŀ��ȿ�֪KIO3��5KI����������غ�ɵã�KIO3+5KI+3H2SO4=3K2SO4+3I2+3H2O������KIO3Ϊ��������
�ʴ�Ϊ��1��5��3��3��3��3��KIO3��
��4����Ӧ�Ĺ�ϵʽΪ��KIO3��3I2��6S2O32-��n��Na2S2O3��=2.0��10-3mol?L-1��0.01L=2.0��10-5mol��
���ԣ�n��KIO3��=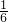 ��n��Na2S2O3��=
��n��Na2S2O3��=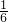 ��2.0��10-5mol��
��2.0��10-5mol��
m��I��=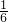 ��2.0��10-5mol��127g/mol=42.3��10-5g��
��2.0��10-5mol��127g/mol=42.3��10-5g��
����wkgʳ���к���42.3��10-5��106mg=423mg��
���������е�ĺ����� mg/kg��
mg/kg��
�ʴ�Ϊ�� ��
��
��������1������ʳ�õ��εĴ��淽����ʳ�÷����Ʋ���ܾ��е����ʣ�
��2�����ʱ������Ϊ���Ե缫��
��3������������ԭ��Ӧ��ѭ�����غ�������غ������ƽ��
��4�����ݷ�Ӧ�Ĺ�ϵʽ��KIO3��3I2��6S2O32-�����м��㣮
���������⿼���Ϊ�ۺϣ��漰Ԫ�ػ�����֪ʶ��������ԭ��Ӧ���绯ѧ�Լ����ʵĺ����ⶨ����Ŀ�ѶȽϴ���ע������й�ϵʽ��Ӧ�ã�
B������ֻ�ܼ���ⵥ�ʣ������ز���Ӧ����B����
C���ɡ����ʱ����ʳƷ��������Ρ�֪���»ᵼ�µ����ʧ����C��ȷ��
D���ɵ�ĺ���35��15mg/kg���õ����е���غ���ԼΪ20��
 ��50��
��50�� ����34��84mg/kg����D��ȷ��
����34��84mg/kg����D��ȷ���ʴ�Ϊ��CD��
��2���ɵ�ⷽ��ʽ��֪���ʱKI��������������˵������Ϊ���Ե缫���ϣ�ӦΪʯī���ʴ�Ϊ��ʯī��
��3����Ӧ��KIO3��KI֮�䷢��������ԭ��Ӧ����I2����������������ԭ��֮���ʧ������Ŀ��ȿ�֪KIO3��5KI����������غ�ɵã�KIO3+5KI+3H2SO4=3K2SO4+3I2+3H2O������KIO3Ϊ��������
�ʴ�Ϊ��1��5��3��3��3��3��KIO3��
��4����Ӧ�Ĺ�ϵʽΪ��KIO3��3I2��6S2O32-��n��Na2S2O3��=2.0��10-3mol?L-1��0.01L=2.0��10-5mol��
���ԣ�n��KIO3��=
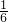 ��n��Na2S2O3��=
��n��Na2S2O3��=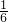 ��2.0��10-5mol��
��2.0��10-5mol��m��I��=
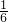 ��2.0��10-5mol��127g/mol=42.3��10-5g��
��2.0��10-5mol��127g/mol=42.3��10-5g������wkgʳ���к���42.3��10-5��106mg=423mg��
���������е�ĺ�����
 mg/kg��
mg/kg���ʴ�Ϊ��
 ��
����������1������ʳ�õ��εĴ��淽����ʳ�÷����Ʋ���ܾ��е����ʣ�
��2�����ʱ������Ϊ���Ե缫��
��3������������ԭ��Ӧ��ѭ�����غ�������غ������ƽ��
��4�����ݷ�Ӧ�Ĺ�ϵʽ��KIO3��3I2��6S2O32-�����м��㣮
���������⿼���Ϊ�ۺϣ��漰Ԫ�ػ�����֪ʶ��������ԭ��Ӧ���绯ѧ�Լ����ʵĺ����ⶨ����Ŀ�ѶȽϴ���ע������й�ϵʽ��Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ
 2011��3�£��ձ�������9.0������������Х�����´��ں��ߵĸ����˵�վ����ϵ�б�ը����Ϊ����ϵͳ�ĺ˷�Ӧ�ѵĵ�һ�㻤��������Ͻ���ǣ��ڶ��㻤����ѹ���֣���һ����̽�ʵ�Ĵ�����������㻤���������̵ĸֺͻ������Ƴɵķdz�������壮��������������ǣ�������
2011��3�£��ձ�������9.0������������Х�����´��ں��ߵĸ����˵�վ����ϵ�б�ը����Ϊ����ϵͳ�ĺ˷�Ӧ�ѵĵ�һ�㻤��������Ͻ���ǣ��ڶ��㻤����ѹ���֣���һ����̽�ʵ�Ĵ�����������㻤���������̵ĸֺͻ������Ƴɵķdz�������壮��������������ǣ�������