��Ŀ����
���Т١���Ϊ���ֶ�����Ԫ�صĻ��ϼۼ�ԭ�Ӱ뾶
��B��C��Ԫ�ؿ��γɻ�����CB2��CB3�����߾��������Ʊ��������
��A��B��Ԫ�ؿ��γɻ�����AB��AB2�����߾��������Ʊ��������ң�
A��B��C��Ϊ�ϱ��е�Ԫ�أ��ס���Ϊ��ѧ����ǿ�ᣮ
��ش��������⣨1��д��BԪ��λ�����ڱ��е�λ��
��2��AԪ����̬�⻯��ĽṹʽΪ
 ��д������Ԫ���γɵ����ȶ����⻯��ĵ���ʽ
��д������Ԫ���γɵ����ȶ����⻯��ĵ���ʽ
 _
_
��3�����й������ʼס��ҵ�˵������ȷ����
a�����ߵ�Ũ��Һ�ڳ���ʱ������������������
b�����ߵ�Ũ��Һ�ڳ��������з��ã���������仯
c�����ߵ�Ũ��Һ�ڳ���ʱ����ͭ��Ӧ
d�����ߵ�ϡ��Һ������ǿ������
��4����д����ҵ����CB2�Ʊ�CB3�Ļ�ѧ����ʽ
д����ҵ����ǿ���ҵĹ������Ʊ�������AB�Ļ�ѧ����ʽ
| Ԫ������ | Ԫ�ر�� | |||||
| �� | �� | �� | �� | �� | �� | |
| ԭ�Ӱ뾶/nm | 0.102 | 0.110 | 0.074 | 0.075 | 0.071 | 0.099 |
| ����ϼ� | +6 | +5 | +5 | +7 | ||
| ��ͻ��ϼ� | -2 | -3 | -2 | -3 | -1 | -1 |
��A��B��Ԫ�ؿ��γɻ�����AB��AB2�����߾��������Ʊ��������ң�
A��B��C��Ϊ�ϱ��е�Ԫ�أ��ס���Ϊ��ѧ����ǿ�ᣮ
��ش��������⣨1��д��BԪ��λ�����ڱ��е�λ��
�ڶ����ڵڢ�A��
�ڶ����ڵڢ�A��
��2��AԪ����̬�⻯��ĽṹʽΪ




��3�����й������ʼס��ҵ�˵������ȷ����
ab
ab
������ĸ��a�����ߵ�Ũ��Һ�ڳ���ʱ������������������
b�����ߵ�Ũ��Һ�ڳ��������з��ã���������仯
c�����ߵ�Ũ��Һ�ڳ���ʱ����ͭ��Ӧ
d�����ߵ�ϡ��Һ������ǿ������
��4����д����ҵ����CB2�Ʊ�CB3�Ļ�ѧ����ʽ
2SO2+O2
2SO3
| ||
| �� |
2SO2+O2
2SO3
��
| ||
| �� |
д����ҵ����ǿ���ҵĹ������Ʊ�������AB�Ļ�ѧ����ʽ
4NH3+5O2
4NO+6H2O
| ||
| ���¸�ѹ |
4NH3+5O2
4NO+6H2O
��
| ||
| ���¸�ѹ |
���������ɱ��ж�����Ԫ�آ١���IJ������ʿ�֪������-2��+6����ֻ��-2�ۣ����ΪS����ΪO���ڢܾ���+5��-3�ۣ�Ϊ�ڢ�A��Ԫ�أ����ԭ�Ӱ뾶��֪����ΪP����ΪN�������Ϊ+7����ͼ�Ϊ-1���ʢ�ΪCl����ֻ��-1�ۣ����ΪFԪ�أ�
A��B��C��Ϊ�ϱ��е�Ԫ�أ��ס���Ϊ��ѧ����ǿ�
��B��C��Ԫ�ؿ��γɻ�����CB2��CB3��ΪSO2��SO3�����߾��������Ʊ�������ף���Ϊ���ᡢBΪ��Ԫ�ء�CΪ��Ԫ�أ�
��A��B��Ԫ�ؿ��γɻ�����AB��AB2����ΪNO��NO2�����߾��������Ʊ��������ң���Ϊ���ᡢAΪ��Ԫ�أ�
Ȼ����Ԫ�ؼ��䵥�ʡ�����������������
A��B��C��Ϊ�ϱ��е�Ԫ�أ��ס���Ϊ��ѧ����ǿ�
��B��C��Ԫ�ؿ��γɻ�����CB2��CB3��ΪSO2��SO3�����߾��������Ʊ�������ף���Ϊ���ᡢBΪ��Ԫ�ء�CΪ��Ԫ�أ�
��A��B��Ԫ�ؿ��γɻ�����AB��AB2����ΪNO��NO2�����߾��������Ʊ��������ң���Ϊ���ᡢAΪ��Ԫ�أ�
Ȼ����Ԫ�ؼ��䵥�ʡ�����������������
����⣺���ɱ��ж�����Ԫ�آ١���IJ������ʿ�֪������-2��+6����ֻ��-2�ۣ����ΪS����ΪO���ڢܾ���+5��-3�ۣ�Ϊ�ڢ�A��Ԫ�أ����ԭ�Ӱ뾶��֪����ΪP����ΪN�������Ϊ+7����ͼ�Ϊ-1���ʢ�ΪCl����ֻ��-1�ۣ����ΪFԪ�أ�
A��B��C��Ϊ�ϱ��е�Ԫ�أ��ס���Ϊ��ѧ����ǿ�
��B��C��Ԫ�ؿ��γɻ�����CB2��CB3��ΪSO2��SO3�����߾��������Ʊ�������ף���Ϊ���ᡢBΪ��Ԫ�ء�CΪ��Ԫ�أ�
��A��B��Ԫ�ؿ��γɻ�����AB��AB2����ΪNO��NO2�����߾��������Ʊ��������ң���Ϊ���ᡢAΪ��Ԫ�أ�
��1��BΪ��Ԫ�أ����ڵڶ����ڵڢ�A�壬
�ʴ�Ϊ���ڶ����ڵڢ�A�壻
��2��AԪ����̬�⻯��ΪNH3����ṹʽΪ ��
��
����Ԫ����FԪ�طǽ�������ǿ��HF���ȶ��������ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
��3��a�������£�Fe��Ũ���ᡢŨ���ᷢ���ۻ������ߵ�Ũ��Һ�ڳ���ʱ�����������������棬��a��ȷ��
b��Ũ���������ˮ�ԣ��ڳ��������з��ã���������Ũ������лӷ��ԣ��ڳ��������з��ã�������С����b��ȷ��
c��Ũ�����ڳ���ʱ��ͭ��Ӧ��Ũ������Ҫ������ͭ��Ӧ����c����
d����ϡ�������ǿ�����ԣ�ϡ����û��ǿ�����ԣ���d����
�ʴ�Ϊ��ab��
��4����ҵ����SO2�Ʊ�SO3�Ļ�ѧ����ʽΪ��2SO2+O2
2SO3��
��ҵ��������Ĺ������Ʊ�������NO�Ļ�ѧ����ʽΪ��4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��2SO2+O2
2SO3��4NH3+5O2
4NO+6H2O��
A��B��C��Ϊ�ϱ��е�Ԫ�أ��ס���Ϊ��ѧ����ǿ�
��B��C��Ԫ�ؿ��γɻ�����CB2��CB3��ΪSO2��SO3�����߾��������Ʊ�������ף���Ϊ���ᡢBΪ��Ԫ�ء�CΪ��Ԫ�أ�
��A��B��Ԫ�ؿ��γɻ�����AB��AB2����ΪNO��NO2�����߾��������Ʊ��������ң���Ϊ���ᡢAΪ��Ԫ�أ�
��1��BΪ��Ԫ�أ����ڵڶ����ڵڢ�A�壬
�ʴ�Ϊ���ڶ����ڵڢ�A�壻
��2��AԪ����̬�⻯��ΪNH3����ṹʽΪ
 ��
������Ԫ����FԪ�طǽ�������ǿ��HF���ȶ��������ʽΪ
 ��
���ʴ�Ϊ��
 ��
�� ��
����3��a�������£�Fe��Ũ���ᡢŨ���ᷢ���ۻ������ߵ�Ũ��Һ�ڳ���ʱ�����������������棬��a��ȷ��
b��Ũ���������ˮ�ԣ��ڳ��������з��ã���������Ũ������лӷ��ԣ��ڳ��������з��ã�������С����b��ȷ��
c��Ũ�����ڳ���ʱ��ͭ��Ӧ��Ũ������Ҫ������ͭ��Ӧ����c����
d����ϡ�������ǿ�����ԣ�ϡ����û��ǿ�����ԣ���d����
�ʴ�Ϊ��ab��
��4����ҵ����SO2�Ʊ�SO3�Ļ�ѧ����ʽΪ��2SO2+O2
| ||
| �� |
��ҵ��������Ĺ������Ʊ�������NO�Ļ�ѧ����ʽΪ��4NH3+5O2
| ||
| ���¸�ѹ |
�ʴ�Ϊ��2SO2+O2
| ||
| �� |
| ||
| ���¸�ѹ |
���������⿼��Ԫ�����ڱ���Ԫ�������ɡ����û�ѧ���Ũ������Ũ�������ʡ���ѧ��ҵ�ȣ��ѶȲ������ñ���Ԫ�ص��������ƶ�Ԫ���ǽ����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
����Dz��ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۣ����ݱ�����Ϣ���ж�����������ȷ���ǣ�������
|
 ����������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��
����������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��CH3COOH+C2H5OH
| ||
| �� |
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2?6C2H5OH��
�ڲ����л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е㣨�棩 | 34.5 | 78.5 | 117.9 | 77 |
��1��Ũ�����������
��2������ͼ��ʾװ�����Ʊ�������������������������ƫ�ͣ���ԭ�����Ϊ
��3��ʵ�����õ������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ���ȥ
��4��Ŀǰ�Ը÷�Ӧ�Ĵ����������µ�̽����������������������Һ��������˷�Ӧ�Ĵ����������ظ�ʹ�ã�ʵ���������±���ʾ��������Ҵ��Ե����ʵ�����ϣ���
| ͬһ��Ӧʱ�� | ͬһ��ӦӦ�¶� | ||||
| ��Ӧ�¶�/�� | ת���ʣ�%�� | ѡ���ԣ�%��* | ��Ӧʱ��/h | ת���ʣ�%�� | ѡ���ԣ�%��* |
| 40 | 77.8 | 100 | 2 | 80.2 | 100 |
| 60 | 92.3 | 100 | 3 | 87.8 | 100 |
| 80 | 92.6 | 100 | 4 | 92.3 | 100 |
| 120 | 94.5 | 98.7 | 6 | 93.0 | 100 |
| *ѡ����100%��ʾ��Ӧ���ɵIJ���ȫ��������������ˮ | |||||
a.120�棬4hb.80�棬2hc.60�棬4hd.40�棬3h
�ڵ���Ӧ�¶ȴﵽ120��ʱ����Ӧѡ���Խ��͵�ԭ�����Ϊ
��ҵβ���е�������ͨ�����ð������շ�����ԭ����NH3��NOx�ڴ��������·�Ӧ�����������ʣ�ijУ�С��ͬѧ��������װ�úͲ���ģ�ҵ�ϵ�������Ĵ������̣�
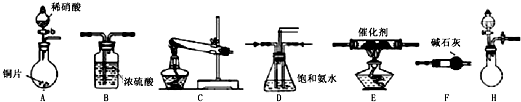
��̽����ȡNH3�ķ���
��1��������װ���У�H�ܿ��١������ȡNH3��װ������Ҫ���ӵķ�Ӧ�Լ�Ϊ ��
��2��Ϊ̽�����õ�ʵ��Ч�����С��ͬѧ��������Cװ������ȡ�������ڿ���ʵ��������ͬ������£�����±���ʵ�����ݣ�
�����������ݣ�����Ϊ���ַ�����ȡ������Ч����ã�����ţ����Ӹ÷���ѡ���ԭ�Ϸ�������Ч���õĿ���ԭ���� ��
��ģ��β������
�С��ͬѧѡ����������װ�ã�������˳�����ӳ�ģ��β������װ�ý���ʵ�飮
��1���������װ����ѡ������Ϊ�����Ľ��в��䣨��ѡװ�ò����ظ�����

��2��A�з�Ӧ�����ӷ���ʽΪ ��
��3��Dװ�õ������У�ʹ�����Ͼ��ȡ����������ٶȡ� ��
��4��Dװ���е�Һ�廹�ɻ��� ������ţ���
a��H2O b��CCl4 c��ŨH2SO4 d��CuSO4��Һ
��5����С��ͬѧ����Ƶ�ģ��β������װ���л�����һ�����Ե�ȱ���� ��

��̽����ȡNH3�ķ���
��1��������װ���У�H�ܿ��١������ȡNH3��װ������Ҫ���ӵķ�Ӧ�Լ�Ϊ
��2��Ϊ̽�����õ�ʵ��Ч�����С��ͬѧ��������Cװ������ȡ�������ڿ���ʵ��������ͬ������£�����±���ʵ�����ݣ�
| �Լ������� | �����Լ� | NH3�����mL�� | |
| a | 6.0g Ca��OH��2�������� | 5.4g NH4Cl | 1344 |
| b | 5.4g ��NH4��2SO4 | 1364 | |
| c | 6.0g NaOH�������� | 5.4g NH4Cl | 1568 |
| d | 5.4g ��NH4��2SO4 | 1559 | |
| e | 6.0g CaO�������� | 5.4g NH4Cl | 1753 |
| f | 5.4g ��NH4��2SO4 | 1792 | |
��ģ��β������
�С��ͬѧѡ����������װ�ã�������˳�����ӳ�ģ��β������װ�ý���ʵ�飮
��1���������װ����ѡ������Ϊ�����Ľ��в��䣨��ѡװ�ò����ظ�����

��2��A�з�Ӧ�����ӷ���ʽΪ
��3��Dװ�õ������У�ʹ�����Ͼ��ȡ����������ٶȡ�
��4��Dװ���е�Һ�廹�ɻ���
a��H2O b��CCl4 c��ŨH2SO4 d��CuSO4��Һ
��5����С��ͬѧ����Ƶ�ģ��β������װ���л�����һ�����Ե�ȱ����

