��Ŀ����
 ��1������VSEPRģ�ͣ�
��1������VSEPRģ�ͣ�ClO3-�ļ۲���ӶԵļ��ηֲ���
CH2Cl2�ļ۲���ӶԵļ��ηֲ���
��2����Ҫ��д���ɵ�������Ԫ��Ϊ����ԭ�ӣ�ͨ��sp3�ӻ��γ����Է��ӵĻ�ѧʽ��
����дһ�֣����������η���
��3����SO42-��Ϊ�ȵ������������������
��4�������Ƶľ�����ͼ��ʾ��ʵ�����Ƶ��ܶ�Ϊd��g?cm-3������֪�Ƶ����ԭ������ΪM�������ӵ�����ΪNA��mol-1�����ٶ�������ԭ��Ϊ�Ⱦ��ĸ������Ҵ�����Խ����ϵ����������У�����ԭ�ӵİ뾶r��cm��Ϊ
���㣺�����ļ���,�жϼ��ӻ����ӵĹ���
ר�⣺��ѧ���뾧��ṹ
��������1�����ݼ۲���ӶԸ���ȷ�����ռ乹�ͣ��۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ�����
��2���ɵ�������Ԫ��Ϊ����ԭ�ӣ�ͨ��sp3�ӻ��γ����Է��ӣ��۲���ӶԸ���Ϊ4�Ҳ����µ��ӶԵķ���Ϊ��������ṹ���۲���ӶԸ���Ϊ4�Һ���һ���µ��ӶԵķ���Ϊ�����νṹ���۲���ӶԸ�����4�Һ��������µ��ӶԵķ���ΪV�η��ӣ�
��3��ԭ�Ӹ�����ȡ��۲��������ȵ�����Ϊ�ȵ����壻
��4���þ�����Naԭ�Ӹ���=1+
��8=2����Naԭ�Ӱ뾶Ϊr�������ı߳�Ϊa�����ݴ�����Խ����ϵ����������У���ͼ��ʾ����1��2��3�����У���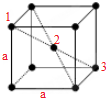 ��4r��2=a2+��
��4r��2=a2+��
a��2��a=
cm���������V=
cm3���ݴ˼�����ԭ�Ӱ뾶��
��2���ɵ�������Ԫ��Ϊ����ԭ�ӣ�ͨ��sp3�ӻ��γ����Է��ӣ��۲���ӶԸ���Ϊ4�Ҳ����µ��ӶԵķ���Ϊ��������ṹ���۲���ӶԸ���Ϊ4�Һ���һ���µ��ӶԵķ���Ϊ�����νṹ���۲���ӶԸ�����4�Һ��������µ��ӶԵķ���ΪV�η��ӣ�
��3��ԭ�Ӹ�����ȡ��۲��������ȵ�����Ϊ�ȵ����壻
��4���þ�����Naԭ�Ӹ���=1+
| 1 |
| 8 |
 ��4r��2=a2+��
��4r��2=a2+��| 2 |
4
| ||
| 3 |
| ||
| �� |
���
�⣺��1��ClO3-�ļ۲���ӶԸ���=3+
����7+1-3��2��=4�Һ���һ���µ��Ӷԣ������伸�ηֲ������������Σ����ӿռ乹��Ϊ�����Σ�
CH2Cl2�ļ۲���ӶԸ���=4�Ҳ����µ��Ӷԣ������伸�ηֲ������������Σ����ӵĿռ�ṹ���������Σ�
�ʴ�Ϊ���������壻�������������壻�����壻
��2���ɵ�������Ԫ��Ϊ����ԭ�ӣ�ͨ��sp3�ӻ��γ����Է��ӣ��۲���ӶԸ���Ϊ4�Ҳ����µ��ӶԵķ���Ϊ��������ṹ��������������ṹ�ķ���ΪSiH4���۲���ӶԸ���Ϊ4�Һ���һ���µ��ӶԵķ���Ϊ�����νṹ�����������νṹ�ķ���ΪPH3���۲���ӶԸ�����4�Һ��������µ��ӶԵķ���ΪV�η��ӣ�����V�η��ӵ�Ϊ H2S��
�ʴ�Ϊ��SiH4��PH3�� H2S��
��3��SO42-��ԭ�Ӹ�����5���۲���Ӹ�����32������������ӻ�Ϊ�õ����������ClO4-��SiO44-���ʴ�Ϊ��ClO4-��SiO44-��
��4���þ�����Naԭ�Ӹ���=1+
��8=2����Naԭ�Ӱ뾶Ϊrcm�������߳�=
cm�������V=��
��3cm3���������V=
cm3=
cm3=��
��3cm3��r=
cm��
�ʴ�Ϊ��
��
| 1 |
| 2 |
CH2Cl2�ļ۲���ӶԸ���=4�Ҳ����µ��Ӷԣ������伸�ηֲ������������Σ����ӵĿռ�ṹ���������Σ�
�ʴ�Ϊ���������壻�������������壻�����壻
��2���ɵ�������Ԫ��Ϊ����ԭ�ӣ�ͨ��sp3�ӻ��γ����Է��ӣ��۲���ӶԸ���Ϊ4�Ҳ����µ��ӶԵķ���Ϊ��������ṹ��������������ṹ�ķ���ΪSiH4���۲���ӶԸ���Ϊ4�Һ���һ���µ��ӶԵķ���Ϊ�����νṹ�����������νṹ�ķ���ΪPH3���۲���ӶԸ�����4�Һ��������µ��ӶԵķ���ΪV�η��ӣ�����V�η��ӵ�Ϊ H2S��
�ʴ�Ϊ��SiH4��PH3�� H2S��
��3��SO42-��ԭ�Ӹ�����5���۲���Ӹ�����32������������ӻ�Ϊ�õ����������ClO4-��SiO44-���ʴ�Ϊ��ClO4-��SiO44-��
��4���þ�����Naԭ�Ӹ���=1+
| 1 |
| 8 |
4
| ||
| 3 |
4
| ||
| 3 |
| ||
| �� |
| 2M |
| NAd |
4
| ||
| 3 |
| 3 |
| ||||
�ʴ�Ϊ��
| 3 |
| ||||
���������⿼���˾����ļ��㡢���ռ乹�͵��жϵ�֪ʶ�㣬���þ�̯�����ܶȹ�ʽ���۲���ӶԻ������۷������ע�⾧�����IJ���ԭ�����У�Ϊ�״��㣮

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ

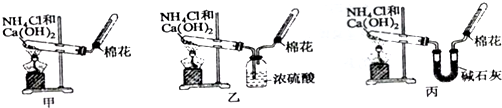
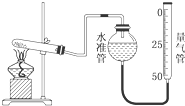 ijѧ�����ø�����طֽ��������ķ�Ӧ���ⶨ�����µ�����Ħ�������ʵ��װ����ͼ��ʾ������ʵ�鲽�裺��װ��ʵ��װ�ã��ڡ��۰������ĸ�����ط�ĩ���������Թ��У�ȷ�����Թܺ�����ط�ĩ������Ϊa g���ܼ��ȣ���ʼ��Ӧ��ֱ������һ���������壮��ֹͣ���ȣ������ռ�����������������ȷ�����ԹܺͲ����������Ϊb g�������ʵ���ҵ��¶ȣ��ش��������⣮
ijѧ�����ø�����طֽ��������ķ�Ӧ���ⶨ�����µ�����Ħ�������ʵ��װ����ͼ��ʾ������ʵ�鲽�裺��װ��ʵ��װ�ã��ڡ��۰������ĸ�����ط�ĩ���������Թ��У�ȷ�����Թܺ�����ط�ĩ������Ϊa g���ܼ��ȣ���ʼ��Ӧ��ֱ������һ���������壮��ֹͣ���ȣ������ռ�����������������ȷ�����ԹܺͲ����������Ϊb g�������ʵ���ҵ��¶ȣ��ش��������⣮