��Ŀ����
���и���������ָ����Һ���ܴ���������ǣ�������
�ٳ����£�c��H+��/c��OH-��=1��10-12����Һ��K+��AlO2-��CO32-��Na+
�ڼ��뱽������ɫ����Һ��K+��NH4+��Cl-��I-
��������Һ��Fe3+��Al3+��NO3-��SO42-
��ʹpH��ֽ��������Һ�У�NH4+��Na+��SO42-��Cl-
����ɫ��Һ�У�K+��Al3+��NO3-��HCO3-��
�ٳ����£�c��H+��/c��OH-��=1��10-12����Һ��K+��AlO2-��CO32-��Na+
�ڼ��뱽������ɫ����Һ��K+��NH4+��Cl-��I-
��������Һ��Fe3+��Al3+��NO3-��SO42-
��ʹpH��ֽ��������Һ�У�NH4+��Na+��SO42-��Cl-
����ɫ��Һ�У�K+��Al3+��NO3-��HCO3-��
| A���ڢ� | B���٢� | C���� | D���٢ܢ� |
�ٳ����£�c��H+��/c��OH-��=1��10-12����Һ�У�c��OH-��=0.1mol/L����Һ�Լ��ԣ���������֮��IJ�������Ӧ���������ܹ��棬�ʢ���ȷ��
�ڼ��뱽������ɫ����Һ�д���Fe3+��Fe3+��I-����������ԭ��Ӧ�������Ӳ��ܴ������棬�ʢڴ���
��Fe3+��pH=4.3��ת��Ϊ��������������Һ�в������Fe3+���ʢ۴���
��ʹpH��ֽ��������Һ���Լ��ԣ�NH4+��OH-�������������ʣ����ܹ��棬�ʢܴ���
��Al3+��HCO3-��ٽ�ˮ�⣬�����Ӳ��ܴ������棬�ʢݴ���
��ѡC��
�ڼ��뱽������ɫ����Һ�д���Fe3+��Fe3+��I-����������ԭ��Ӧ�������Ӳ��ܴ������棬�ʢڴ���
��Fe3+��pH=4.3��ת��Ϊ��������������Һ�в������Fe3+���ʢ۴���
��ʹpH��ֽ��������Һ���Լ��ԣ�NH4+��OH-�������������ʣ����ܹ��棬�ʢܴ���
��Al3+��HCO3-��ٽ�ˮ�⣬�����Ӳ��ܴ������棬�ʢݴ���
��ѡC��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��Na+��HSO
��Na+��HSO ��PO
��PO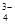 ��MnO
��MnO
 ��NO
��NO ��SO
��SO
 ��CO
��CO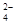 ��Cl
��Cl =0.1 mol/L����Һ�У�Na+��K+��SO
=0.1 mol/L����Һ�У�Na+��K+��SO