��Ŀ����
12��ʵ���Һϳ����������IJ������£���Բ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý���������ͼ1��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺
����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩
��1������ƿ�г��˼����Ҵ���Ũ����������⣬��Ӧ�������Ƭ����Ŀ���Ƿ�ֹ���У�
��2������ƿ�м���һ���������Ҵ���Ũ����Ļ��Һ�ķ����ǣ�������ƿ�м���һ�������Ҵ���Ȼ��������Ũ���������ƿ���ӱ���
��3���ڸ�ʵ���У�����1mol�Ҵ���1mol������Ũ���������¼��ȣ���ַ�Ӧ���ܷ�����1mol����������Ϊʲô������Ϊ�÷�Ӧ�ǿ��淴Ӧ����Ӧ���ܽ�����ȫ��
��4��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ2�Ƿ��������������ͼ������ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����
�Լ�a�DZ���Na2CO3��Һ���Լ�b��������뷽�����Ƿ�Һ�����뷽�����������뷽����������
��5���ڵõ���A�м�����ˮ̼���Ʒ�ĩ����Ŀ���dz�ȥ���������л��е�����ˮ��
���� ��1��ʵ���м���Һ����ܷ������У�
��2��Ũ�����ܶȴ�Ӧ��Ũ������뵽�Ҵ��У��Է���Һ�ɽ���
��3��������Ҵ��������������ķ�Ӧ���ڿ��淴Ӧ��
��4����ʵ�����̿�֪��aΪ����̼������Һ�����뷽��IΪ��Һ�����Ͳ�AΪ��������������õ�AΪ����������ˮ��B�������ơ�̼���ơ��Ҵ������뷽��IIΪ���õ�EΪ�Ҵ���C�к������ơ�̼���ƣ��Լ�bΪ���ᣬD�к������ơ����ᣬ���뷽����Ϊ����
��5����ˮ̼���Ʒ�ĩ��һ�����õ���ˮ�������Գ������������л��е�����ˮ��
��� �⣺��1������ƿ�г��˼����Ҵ���Ũ����������⣬��Ӧ�������Ƭ��Ŀ���Ƿ�ֹ���У��ʴ�Ϊ�����Ƭ����ֹ���У�
��2��Ũ�����ܶȴ�Ϊ��ֹ��Һ�ɽ���Ӧ��Ũ�����������뵽�ܶ�С���Ҵ��У��ӱ���
�ʴ�Ϊ��������ƿ�м���һ�������Ҵ���Ȼ��������Ũ���������ƿ���ӱ���
��3��������Ҵ��������������ķ�Ӧ���ڿ��淴Ӧ����Ӧ���ܽ�����ȫ�����Բ�������1mol�����������ʴ�Ϊ������Ϊ�÷�Ӧ�ǿ��淴Ӧ����Ӧ���ܽ�����ȫ��
��4����ȥ���������е��Ҵ��������ñ���Na2CO3��Һ����Һ�ֲ㣬�÷�Һ�ķ������룬A������������B�������ƺ��Ҵ��Ļ����Һ��������ķ������룬E���Ҵ���C�������ƣ�Ҫ�õ�����ͼ���ǿ�ᣬ�����Լ�B�����ᣮ���ݷ������Լ�a�DZ���Na2CO3��Һ���Լ�b��������뷽�����Ƿ�Һ�����뷽�����������뷽���������ʴ�Ϊ������Na2CO3��Һ�������Һ����������
��5���õ������������л���������ˮ�����Լ�����ˮ̼���Ʒ�ĩ����Ŀ���dz�ȥ���������л��е�����ˮ���ʴ�Ϊ����ȥ���������л��е�����ˮ��
���� ���⿼���л�����Ʊ������������ᴿ��Ϊ��Ƶ���㣬���������еķ�Ӧ���������뷽��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע���л�������ʣ���Ŀ�ѶȲ���

 ͼ��ʵ����������������װ�ã�
ͼ��ʵ����������������װ�ã���1������ǰ��ͨ����Ҫ���Թܢ��м������Ƭ��Ŀ���Ƿ�ֹ���У�
��2��Ϊ��֤��Ũ��������ã�ijͬѧ����������4��ʵ�飬ʵ���¼�����
| ʵ�� ��� | �Թܢ����Լ� | �Թܢ����Լ� | �л�����/cm |
| A | 3mL�Ҵ���2mL���ᡢ1mL18mol•L-1Ũ���� | ����Na2CO3��Һ | 5.0 |
| B | 3mL�Ҵ���2mL���� | ����Na2CO3��Һ | 0.1 |
| C | 3mL�Ҵ���2mL���ᡢ6mL3mol•L-1���� | ����Na2CO3��Һ | 1.2 |
| D | 3mL�Ҵ���2mL���ᡢ6mL6mol•L-1���� | ����Na2CO3��Һ | 1.2 |
�ڷ���ʵ��A��B��C�����ݣ����Եó�Ũ�����ڷ�Ӧ�е������Ǵ�������ˮ����ʵ��D��ʵ��C���գ������ܵó��Ľ����ǶԸ÷�Ӧ������õ�ʵ����ΪH+��
��3������Na2CO3��Һ���������к����ᡢ�ܽ��Ҵ�����������ˮ�е��ܽ�ȣ����ֲ㣮

��1������ʯ��������ܽ����Һ�����Mg2+�⣬�����еĽ���������Fe3+��Al3+�������ӷ��ţ���
��2�����Т����ʱ��������Һ��pH=7-8���й��������������pH���±���Ca��OH��2���ܹ�������Ca��OH��2�������ܻᵼ��Al��OH��3���ѧʽ����ͬ���ܽ⡢��Mg��OH��2������
| �������� | Fe��OH��3 | Al��OH��3 | Mg��OH��2 |
| ��ʼ����pH | 2.3 | 3.3 | 9.4 |
��4������ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ�������CaCO3��CO2��
��5�������һ��ʵ�飬ȷ����ƷaMgCO3•bMg��OH��2•cH2O��a��b��c��ֵ�����������ʵ�鲽�裨�����Լ���Ũ���ᡢ��ʯ�ң���
�ٳ�����Ʒ���ڸ��·ֽ⣬����Ũ��������ˮ�������ܳ�����ʯ������CO2ǰ����������ݳƳ�MgO��
��.18.2g��Ʒ��ȫ�ֽ����6.6gCO2��8.0gMgO���ɴ˿�֪����Ʒ�Ļ�ѧʽ�У�a=3��b=1��c=3��
| A�� | CH3CHO | B�� | CH2�TCHCOOH | C�� | CH3CH2OH | D�� | CH2�TCHCHO |
 ��ʵ�������ǿ�������ͼ��ʾ��װ������ȡ�����������Իش��������⣺
��ʵ�������ǿ�������ͼ��ʾ��װ������ȡ�����������Իش��������⣺ �����dzµ��㡱������Ϊ���ڴ������������������ζ��������������ʵ�������ǿ�������ͼ��ʾ��װ������ȡ������������ش��������⣺
�����dzµ��㡱������Ϊ���ڴ������������������ζ��������������ʵ�������ǿ�������ͼ��ʾ��װ������ȡ������������ش��������⣺ �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺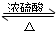 CH3COOC2H5+H2O��
CH3COOC2H5+H2O�� ��
�� �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺