��Ŀ����
25 �桢101 kPa��
��2Na(s)+1/2O2(g)===Na2O(s) ��H1��-414 kJ/mol
��2Na(s)+O2(g)===Na2O2(s) ��H2��-511 kJ/mol
����˵����ȷ����
- A.�ٺ͢ڲ�����������Ӹ����Ȳ����
- B.�ٺ͢����ɵ����ʵ����IJ��ת�Ƶ�������ͬ
- C.������Na������O2��Ӧ����Na2O�����¶���������Na2O�������ӿ�
- D.25 �桢101 kPa�£�Na2O2(s)+2Na(s)===2Na2O(s) ��H��-317 kJ/mol
D
A����ȷ���ٺ͢ڲ�����������Ӹ�������ȣ�����1�U2�ģ�B����ȷ���ٺ͢����ɵ����ʵ����IJ��ת�Ƶ�������ȣ���Ϊ���ڷ�Ӧ�ж���ʧȥ1�����ӵģ�C����ȷ�����ȵ������£��ƺ������������ɹ������ƣ��ò��������������ݸ�˹���ɿ�֪��ѡ��D��ȷ����ѡD��
���㣺����������ԭ��Ӧ���й��жϡ���������Է�Ӧ���ʵ�Ӱ���Լ���˹���ɵ�Ӧ�õ�
���������жϹ��������е�������ʱ���ܸ��ݻ�ѧʽ����Ҫ���������Ľṹ�ص㣬����������Ƶĵ���ʽ�ȡ�
A����ȷ���ٺ͢ڲ�����������Ӹ�������ȣ�����1�U2�ģ�B����ȷ���ٺ͢����ɵ����ʵ����IJ��ת�Ƶ�������ȣ���Ϊ���ڷ�Ӧ�ж���ʧȥ1�����ӵģ�C����ȷ�����ȵ������£��ƺ������������ɹ������ƣ��ò��������������ݸ�˹���ɿ�֪��ѡ��D��ȷ����ѡD��
���㣺����������ԭ��Ӧ���й��жϡ���������Է�Ӧ���ʵ�Ӱ���Լ���˹���ɵ�Ӧ�õ�
���������жϹ��������е�������ʱ���ܸ��ݻ�ѧʽ����Ҫ���������Ľṹ�ص㣬����������Ƶĵ���ʽ�ȡ�

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�
�����Ŀ
 (aq)+
(aq)+ (aq)+2
(aq)+2 (aq) = BaSO4(s)+2H
(aq) = BaSO4(s)+2H O(l);
O(l);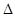 H =
H = 57.3 kJ/mol
57.3 kJ/mol H
H O
O 57.3 kJ/mol
57.3 kJ/mol H2 SO4(aq)=
H2 SO4(aq)= O2(g)=8CO2(g)+9H2O ��H=
O2(g)=8CO2(g)+9H2O ��H= (aq)+
(aq)+ (aq)+2OH
(aq)+2OH (aq)=BaSO4(s)+2H
(aq)=BaSO4(s)+2H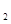 O(1);
O(1); H=
H= 57.3
kJ/mol
57.3
kJ/mol B.KOH(aq)+
B.KOH(aq)+ H
H O(I);
O(I);  O
O