��Ŀ����
���ݻ�Ϊ2L���ܱ������У�������ϵ���¶�Ϊ800�治�䣬��һ������X��Y�����Ϸ�����Ӧ��
2X(g)+Y(g) 2Z(g) ����X�����ʵ���[n(X)]��ʱ��ı仯���±�
2Z(g) ����X�����ʵ���[n(X)]��ʱ��ı仯���±�
2X(g)+Y(g)
 2Z(g) ����X�����ʵ���[n(X)]��ʱ��ı仯���±�
2Z(g) ����X�����ʵ���[n(X)]��ʱ��ı仯���±�
��ش��������⣺
(1)��Y��ʾ��Ӧ��ʼ���ﵽƽ�⣨����30s�ոմﵽƽ�⣩ʱ��ƽ����Ӧ����__________��0~10s��10~20 s�ķ�Ӧ����֮��Ϊ___________��
(2)�����������ݣ��ܷ����800��ʱ�÷�Ӧ��ƽ�ⳣ��K�����ܡ������K=____________��������˵��ȱ�ٵ����ݣ�____________��
(3)�����߷�Ӧ��ϵ���¶ȣ�ʹ��Ӧ���´ﵽƽ�⣬��ʱ��ϵ��n(X)=n(Z)����÷�Ӧ��__________��ѡ�� �����������š����ȷ�Ӧ��
(4)��800��ʱ������С������������ﵽ��ƽ��ʱn(X)___��ѡ�>������=����<����0.07 mol��X��ת����_______��ѡ����������䡱��С������
(5)����800��ʱ�ķ�Ӧ�ﵽƽ��ʱ��÷ų�����akJ����д���ڴ�������X��Y��Ӧ���Ȼ�ѧ����ʽ___________________________��
(1)��Y��ʾ��Ӧ��ʼ���ﵽƽ�⣨����30s�ոմﵽƽ�⣩ʱ��ƽ����Ӧ����__________��0~10s��10~20 s�ķ�Ӧ����֮��Ϊ___________��
(2)�����������ݣ��ܷ����800��ʱ�÷�Ӧ��ƽ�ⳣ��K�����ܡ������K=____________��������˵��ȱ�ٵ����ݣ�____________��
(3)�����߷�Ӧ��ϵ���¶ȣ�ʹ��Ӧ���´ﵽƽ�⣬��ʱ��ϵ��n(X)=n(Z)����÷�Ӧ��__________��ѡ�� �����������š����ȷ�Ӧ��
(4)��800��ʱ������С������������ﵽ��ƽ��ʱn(X)___��ѡ�>������=����<����0.07 mol��X��ת����_______��ѡ����������䡱��С������
(5)����800��ʱ�ķ�Ӧ�ﵽƽ��ʱ��÷ų�����akJ����д���ڴ�������X��Y��Ӧ���Ȼ�ѧ����ʽ___________________________��
(1)0.0011 mol/(L��s)��5:1
(2)ȱ�ٳ�ʼY�����ʵ�������Ũ�ȣ�
(3)��
(4)<������
(5)2X(g)+Y(g) 2Z(g) ��H=-15.4a kJ/mol
2Z(g) ��H=-15.4a kJ/mol
(2)ȱ�ٳ�ʼY�����ʵ�������Ũ�ȣ�
(3)��
(4)<������
(5)2X(g)+Y(g)
 2Z(g) ��H=-15.4a kJ/mol
2Z(g) ��H=-15.4a kJ/mol 
��ϰ��ϵ�д�
 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
�����Ŀ
 ��2011?��������ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1����ش��������⣺
��2011?��������ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1����ش��������⣺ ��֪��Ӧ��CO��g��+H2O��g��?H2��g��+CO2��g����H=-41.2kJ/mol�����ɵ�CO2��H2�Բ�ͬ������Ȼ��ʱ�ں��������µķ�Ӧ���Ƶ�CH4��
��֪��Ӧ��CO��g��+H2O��g��?H2��g��+CO2��g����H=-41.2kJ/mol�����ɵ�CO2��H2�Բ�ͬ������Ȼ��ʱ�ں��������µķ�Ӧ���Ƶ�CH4��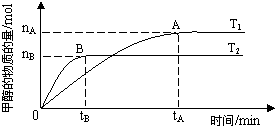 ��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����֪H2��g����CO��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ?mol-1��-283.0kJ?mol-1��-726.5kJ?mol-1��
