��Ŀ����
��ͼ��A��B��C��D��E��F��G��Ϊ�л����������ͼ�ش����⣺

��1��C�����������ŵ������� ��B�ķ���ʽ�� ��
��2����Ӧ�۵Ļ�ѧ����ʽ�� �����л������ýṹ��ʽ��ʾ����
��3��A�Ľṹ��ʽ�� ����Ӧ��������������ȥ��Ӧ���� ��
��4����������3��������B��ͬ���칹�����Ŀ�� ����
��i�������ڶ�ȡ�������ṹ����ii����B����ͬ�Ĺ����ţ���iii������FeCl2��Һ������ɫ��Ӧ��
д����������һ��ͬ���칹��Ľṹ��ʽ ��

��1��C�����������ŵ�������
��2����Ӧ�۵Ļ�ѧ����ʽ��
��3��A�Ľṹ��ʽ��
��4����������3��������B��ͬ���칹�����Ŀ��
��i�������ڶ�ȡ�������ṹ����ii����B����ͬ�Ĺ����ţ���iii������FeCl2��Һ������ɫ��Ӧ��
д����������һ��ͬ���칹��Ľṹ��ʽ
���㣺�л�����ƶ�
ר�⣺
������B��Ũ�����������������E����E�Ľṹ��֪��B����������Ӧ����E����BΪ ��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��GΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ
��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��GΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ ���ݴ˽��
���ݴ˽��
 ��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��GΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ
��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��GΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ ���ݴ˽��
���ݴ˽�����
�⣺B��Ũ�����������������E����E�Ľṹ��֪��B����������Ӧ����E����BΪ ��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��GΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ
��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��GΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ ��
��
��1��CΪCH3COOH��C�����������ŵ��������Ȼ���BΪ ��B�ķ���ʽ��C9H10O3��
��B�ķ���ʽ��C9H10O3��
�ʴ�Ϊ���Ȼ���C9H10O3��
��2����Ӧ����CH3COOH��CH3CH2OH����������Ӧ����������������Ӧ����ʽΪCH3COOH+CH3CH2OH
CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOC2H5+H2O��
��3���������������֪��AΪ ����Ӧ�������У���Ӧ��������ˮ�⣬��Ӧ�ڷ�������������Ӧ������������Ӧ������ȥ��Ӧ������������ȥ��Ӧ���Ǣ٢ڢۣ�
����Ӧ�������У���Ӧ��������ˮ�⣬��Ӧ�ڷ�������������Ӧ������������Ӧ������ȥ��Ӧ������������ȥ��Ӧ���Ǣ٢ڢۣ�
�ʴ�Ϊ�� ���٢ڢۣ�
���٢ڢۣ�
��4�� ��ͬ���칹���������3��������
��ͬ���칹���������3��������
�ٺ����ڶ�ȡ�������ṹ������B����ͬ�����ţ�����-COOH��-OH���۲���FeCl3��Һ������ɫ��Ӧ���������ǻ�������������ͬ���칹��Ϊ��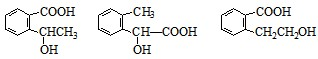 ������3�֣�
������3�֣�
�ʴ�Ϊ��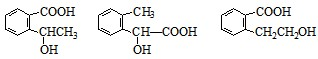 ������һ�֣�
������һ�֣�
 ��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��GΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ
��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��GΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ ��
����1��CΪCH3COOH��C�����������ŵ��������Ȼ���BΪ
 ��B�ķ���ʽ��C9H10O3��
��B�ķ���ʽ��C9H10O3���ʴ�Ϊ���Ȼ���C9H10O3��
��2����Ӧ����CH3COOH��CH3CH2OH����������Ӧ����������������Ӧ����ʽΪCH3COOH+CH3CH2OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3COOH+CH3CH2OH
| Ũ���� |
| �� |
��3���������������֪��AΪ
 ����Ӧ�������У���Ӧ��������ˮ�⣬��Ӧ�ڷ�������������Ӧ������������Ӧ������ȥ��Ӧ������������ȥ��Ӧ���Ǣ٢ڢۣ�
����Ӧ�������У���Ӧ��������ˮ�⣬��Ӧ�ڷ�������������Ӧ������������Ӧ������ȥ��Ӧ������������ȥ��Ӧ���Ǣ٢ڢۣ��ʴ�Ϊ��
 ���٢ڢۣ�
���٢ڢۣ� ��4��
 ��ͬ���칹���������3��������
��ͬ���칹���������3���������ٺ����ڶ�ȡ�������ṹ������B����ͬ�����ţ�����-COOH��-OH���۲���FeCl3��Һ������ɫ��Ӧ���������ǻ�������������ͬ���칹��Ϊ��
 ������3�֣�
������3�֣��ʴ�Ϊ��
 ������һ�֣�
������һ�֣�
���������⿼���л�����ƶϣ�ע�����ת����ϵ��E�Ľṹ�Լ�D��G�ķ���ʽ�����ƶϣ����չ����ŵ�������ת���ǹؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
��״����3.2g O2��2.24L�����������Ȼ������ȵģ�������
| A������ | B��Ħ������ |
| C���������� | D��������� |
�������ӷ���ʽ��ȷ���ǣ�������
| A����Mg�� HC03��2��Һ�м��������NaOH��Һ��Mg2++2 HC03-+20H-�TMgC03��+C032-+2H20 |
| B����NH4 Al�� S04��2��Һ�е���Ba�� OH��2��ҺʹSO42-��Ӧ��ȫ��2Ba2++40H-ʮAl3++2 SO42-�T2BaS04��+Al02-+2H20 |
| C���㹻��C02ͨ�뱥��̼������Һ�У�C02+CO32-+H20=2 HC03- |
| D����Fe�� N03��2��Һ�м���ϡ���3Fe2++4H++N03-�T3Fe3++NO��+2H20 |
������װ�ý�����Ӧʵ�飬�ܴﵽʵ��Ŀ���ǣ�������
A�� ����Cu��ŨH2SO4��Ӧ��ȡ������SO2���� |
B�� �����Ʊ����ռ�NH3 |
C�� ���γ�ԭ��ز�����ȶ����� |
D�� ���ڼ����Ƶõ���ϩ���Ƿ����SO2��CO2 |
 ��ͪ��CH3COCH3����ij�ܼ���ڴ��������·�����Ӧ��2CH3COCH3��aq��?CH3COCH2COH��CH3��2��aq��ȡ��ͬŨ�ȵ�CH3COCH3���ֱ���40���60��ʱ�������ת���ʦ���ʱ��仯�Ĺ�ϵ���ߣ���-t����ͼ��ʾ������˵����ȷ���ǣ�������
��ͪ��CH3COCH3����ij�ܼ���ڴ��������·�����Ӧ��2CH3COCH3��aq��?CH3COCH2COH��CH3��2��aq��ȡ��ͬŨ�ȵ�CH3COCH3���ֱ���40���60��ʱ�������ת���ʦ���ʱ��仯�Ĺ�ϵ���ߣ���-t����ͼ��ʾ������˵����ȷ���ǣ�������| A��b����40��ʱCH3COCH3�Ħ�-t���� |
| B�������¶ȿ����̸÷�Ӧ��ƽ���ʱ�䲢�����ƽ��ת���� |
| C������ѹǿ�����̸÷�Ӧ��ƽ���ʱ�䲢�����ƽ��ת���� |
| D��������Ӧ���淴ӦΪ���ȷ�Ӧ |
������ѧ�Ļ�ѧ֪ʶ�жϣ������йػ�ѧ�������������ģ�������
| A��һ�������£������ƿ��Գ�Ϊ��Ե�� |
| B��һ�������£�ˮ��20��ʱ�����̳ɹ��� |
| C���ø�ͷ��ľ��һ��Ϊ��������������и���ԭ��ǡ�÷ֳɸ�С�� |
| D����ǧ����ǰ������һ���������ڵ�ij��ԭ�ӿ�������������� |
��һ���������۷����Ȼ�����Һ�У���ַ�Ӧ��������Һ��Fe3+��Fe2+��Ũ��ǡ����ȣ���Ӧ������δ��Ӧ��Fe3+�����ʵ���֮��Ϊ��������
| A��1��3 | B��1��2 |
| C��1��1 | D��3��1 |