��Ŀ����
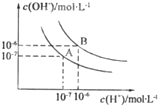 ˮ�ĵ���ƽ��������ͼ��ʾ��
ˮ�ĵ���ƽ��������ͼ��ʾ����1������A���ʾ25��ʱˮ����ƽ��ʱ���ӵ�Ũ�ȣ����¶����ߵ�100��ʱ��ˮ�ĵ���ƽ��״̬�䵽B�㣬���ʱˮ�����ӻ���
��2����֪25��ʱ��0.1L 0.1mol?L-1��NaA��Һ��pH=10����NaA��Һ�������ڵ����ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ
��3��25��ʱ����pH=11��NaOH��Һ��pH=4��������Һ��ϣ������û����ҺpH=9����NaOH��Һ��������Һ�������Ϊ
��4��100��ʱ����10�����ijǿ����Һ��1�����ijǿ����Һ��Ϻ���Һ�����ԣ�����֮ǰ����ǿ���pH��ǿ���pH֮��Ӧ����Ĺ�ϵ��
���㣺ˮ�ĵ���,pH�ļ���,����ˮ���Ӧ��
ר�⣺����ƽ������Һ��pHר��,�����ˮ��ר��
��������1�����ͼʾ����ˮ�����ӻ�����ʽKw=c��H+��?c��OH-��������������Ũ�ȼ����25��ʱ��100��ˮ�����ӻ���ˮ����Ϊ���ȷ�Ӧ�������¶��ܴٽ�ˮ�ĵ��룻
��2������0.1L 0.1mo/L��NaA��Һ��pH=10�ж��ε����ͣ�
��3���������������ҺΪx��������Һ�����Ϊy����������������ʽ��������֮�ȣ�
��4���������Һ��pHΪa������Һ��pHΪb������100��ʱ�������ϵ��ʽ���㣮
��2������0.1L 0.1mo/L��NaA��Һ��pH=10�ж��ε����ͣ�
��3���������������ҺΪx��������Һ�����Ϊy����������������ʽ��������֮�ȣ�
��4���������Һ��pHΪa������Һ��pHΪb������100��ʱ�������ϵ��ʽ���㣮
���
�⣺��1��A��25��ʱ��c��H+��=c��OH-��=1��10-7mol/L��Kw=c��H+��?c��OH-��=1��10-7��1��10-7=10-14��
100��ʱ��c��H+��=c��OH-��=1��10-6mol/L��Kw=c��H+��?c��OH-��=1��10-6��1��10-6=10-12��ˮ����Ϊ���ȷ�Ӧ�������¶��ܴٽ�ˮ�ĵ��룻
�ʴ�Ϊ��10-14��10-12���¶����ߣ�ˮ�ĵ���̶���������Һ����������Ũ�Ⱥ�����������Ũ�����Ӷ�����Kw����
��2������0.1L 0.1mo/L��NaA��Һ��pH=10��˵����Һ��ʾ���ԣ�����ǿ�������Σ��ʲ�ˮ���������Ũ�ȴ���A-����Ũ�ȣ���Һ�Լ��ԣ�������Ũ�ȴ�������������Ũ�ȣ��ʴ�Ϊ��c��Na+����c��A-����c��OH-����c��H+����
��3��������������Һ�����ΪxL��������Һ�����ΪyL��pH=11��NaOH��Һ�У����������ӵ�Ũ��Ϊ��10-3mol/L��pH=4��������Һ��������Ũ��Ϊ��10-4mol/L��
����Ϻ���Һ��pH=9����Һ���Լ��ԣ���Һ�����������ӵ�Ũ��Ϊ10-5mol/L�����������ƹ�������10-3mol/L��xL-10-4mol/L��y=10-5mol/L��x+y����
���x��y=1��9��
�ʴ�Ϊ��1��9��
��4����ǿ����Һ��pHΪa�����Ϊ10V����Һ��������Ũ��Ϊ��10-amol/L������Һ��pHΪb�����ΪV����Һ�����������ӵ�Ũ��Ϊ��10-��12-b��mol/L��
��Ϻ���Һ�����ԣ���������Һ�������ӵ����ʵ����������������ӵ����ʵ�������10-amol/L��10VL=10-��12-b��mol/L��VL��
��ã�1-a=b-12��a+b=13��
�ʴ�Ϊ��pH��+pH��=13��
100��ʱ��c��H+��=c��OH-��=1��10-6mol/L��Kw=c��H+��?c��OH-��=1��10-6��1��10-6=10-12��ˮ����Ϊ���ȷ�Ӧ�������¶��ܴٽ�ˮ�ĵ��룻
�ʴ�Ϊ��10-14��10-12���¶����ߣ�ˮ�ĵ���̶���������Һ����������Ũ�Ⱥ�����������Ũ�����Ӷ�����Kw����
��2������0.1L 0.1mo/L��NaA��Һ��pH=10��˵����Һ��ʾ���ԣ�����ǿ�������Σ��ʲ�ˮ���������Ũ�ȴ���A-����Ũ�ȣ���Һ�Լ��ԣ�������Ũ�ȴ�������������Ũ�ȣ��ʴ�Ϊ��c��Na+����c��A-����c��OH-����c��H+����
��3��������������Һ�����ΪxL��������Һ�����ΪyL��pH=11��NaOH��Һ�У����������ӵ�Ũ��Ϊ��10-3mol/L��pH=4��������Һ��������Ũ��Ϊ��10-4mol/L��
����Ϻ���Һ��pH=9����Һ���Լ��ԣ���Һ�����������ӵ�Ũ��Ϊ10-5mol/L�����������ƹ�������10-3mol/L��xL-10-4mol/L��y=10-5mol/L��x+y����
���x��y=1��9��
�ʴ�Ϊ��1��9��
��4����ǿ����Һ��pHΪa�����Ϊ10V����Һ��������Ũ��Ϊ��10-amol/L������Һ��pHΪb�����ΪV����Һ�����������ӵ�Ũ��Ϊ��10-��12-b��mol/L��
��Ϻ���Һ�����ԣ���������Һ�������ӵ����ʵ����������������ӵ����ʵ�������10-amol/L��10VL=10-��12-b��mol/L��VL��
��ã�1-a=b-12��a+b=13��
�ʴ�Ϊ��pH��+pH��=13��
���������⿼����ˮ�ĵ���ƽ����������ӻ������ļ���Ӧ�ã�ע�����ӻ���һ���¶��µij��������¶ȸı䣬���ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
���й���ͭ�缫��������ȷ���ǣ�������
| A��ͭпԭ�����ͭ������ |
| B���õ�ⷨ����ͭʱ��ͭ������ |
| C���ڵ��ʱ���Ƽ��϶�ͭ����ͭ������ |
| D����ⱥ��ʳ��ˮ��ȡ����ʱ����ͭƬ������ |
 ��ͼ�������Լ�ƿ��ǩ�ϵ�����
��ͼ�������Լ�ƿ��ǩ�ϵ����� ��2����Ӧ������A��g����B��g����C��g�����ʵ����仯��ͼ��ʾ������ͼ����ʾ�ж�����˵����ȷ����
��2����Ӧ������A��g����B��g����C��g�����ʵ����仯��ͼ��ʾ������ͼ����ʾ�ж�����˵����ȷ����