��Ŀ����
��д��ʵ�����ư����Ļ�ѧ����ʽ��______��
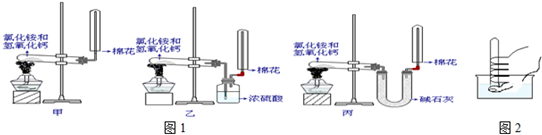
����ͼ��ʾ�ķ����ڻ����������ռ�װ�ã��г�װ�ÿ���ȥ��
��
��1��Ũ������ľ̿���ڼ��������·�Ӧ����֪����KMnO4��Һ��������SO2�����������ʾ�ĸ�װ�����һ��ʵ�飬��֤������Ӧ�������ĸ��ֲ��
| ��� | �� | �� | �� | �� |
| װ�� | 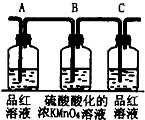 |  | 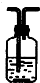 |  |
______��______��______��______��
��2��ʵ��ʱ�ɹ۲쵽װ�â���Aƿ����Һ��ɫ��Cƿ����Һ����ɫ��Aƿ��Һ��������______��Bƿ��Һ��������______��Cƿ��Һ��������______��
��3��װ�â������ӵĹ���ҩƷ��______����ȷ֤�IJ�����______��ȷ��װ�â�������װ����λ�õ�������______��
�⣺��ʵ�������Ȼ�狀����������ڼ�����������ȡ��������Ӧ����ʽΪ��2NH4Cl+Ca��OH��2 CaCl2+2H2O+2NH3���������ͼ�������ˮ�����Բ�������ˮ���ռ��������£���������������Ӧ���Ұ����ܶ�С�ڿ��������Կ��Բ��������ſ������ռ������ռ�װ��ͼΪ��
CaCl2+2H2O+2NH3���������ͼ�������ˮ�����Բ�������ˮ���ռ��������£���������������Ӧ���Ұ����ܶ�С�ڿ��������Կ��Բ��������ſ������ռ������ռ�װ��ͼΪ��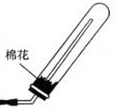 ��
��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2 CaCl2+2H2O+2NH3����
CaCl2+2H2O+2NH3����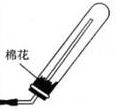 ��
��
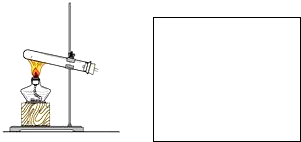
��1�����������̼�Ͷ�������ʱ�õ�����Һ�о�����ˮ�������ȼ���ˮ�Ĵ��ڣ�������̼�Ͷ������������ʹ����ʯ��ˮ����ǣ������ȼ�����������ٳ�ȥ�������������̼����������˳���ǣ��ܡ��ڡ��١���
���ʴ�Ϊ���ܡ��ڡ��١��ۣ�
��2��AΪ�����������װ�ã�B��CΪ��ȥ�����������Ƿ�������������װ�ã�
�ʴ�Ϊ������SO2���壬����SO2���壬����SO2�����Ƿ������
��3����ˮ����ͭΪ��ɫ���壬��ˮ�ɱ�Ϊ��ˮ����ͭ��CuSO4?5H2O��Ϊ��ɫ���壬���ڼ���ˮ�Ĵ��ڣ����ڲ�������ͨ���١���ʱ�����ˮ���������Ԣڱ����ڢ١���֮ǰ��
�ʴ�Ϊ����ˮCuSO4��ˮ���������ڲ�������ͨ���١���ʱ�����ˮ���������Ԣڱ����ڢ١���֮ǰ��
������I��ʵ�������������ƺ��Ȼ����ȡ���������ݰ��������ʼ��ܶ�ȷ���ռ�������
II����1�����������̼�Ͷ�������ʱ�õ�����Һ�о�����ˮ�������ȼ���ˮ�Ĵ��ڣ�������̼�Ͷ������������ʹ����ʯ��ˮ����ǣ������ȼ�����������ٳ�ȥ�������������̼��
��2������ʵ��Ŀ�ĺ�װ��ͼ���Dz���������Լ������ü�������Ӧ�������忼�ǣ�AΪ�����������װ�ã�B��CΪ��ȥ�����������Ƿ�������������װ�ã�
��3������ʵ��Ŀ��֪����Ҫ����ˮ������ˮ������ʹ��ˮ����ͭ���������������������ȷ����ˮ����ͭ��λ�ã�
���������⿼�鰱������ȡ�����ʵļ��飬ע�����������õ��Լ��Լ�������Ⱥ�˳��Ϊ�״��㣬�ѶȽϴ�
 CaCl2+2H2O+2NH3���������ͼ�������ˮ�����Բ�������ˮ���ռ��������£���������������Ӧ���Ұ����ܶ�С�ڿ��������Կ��Բ��������ſ������ռ������ռ�װ��ͼΪ��
CaCl2+2H2O+2NH3���������ͼ�������ˮ�����Բ�������ˮ���ռ��������£���������������Ӧ���Ұ����ܶ�С�ڿ��������Կ��Բ��������ſ������ռ������ռ�װ��ͼΪ�� ��
���ʴ�Ϊ��2NH4Cl+Ca��OH��2
 CaCl2+2H2O+2NH3����
CaCl2+2H2O+2NH3���� ��
����1�����������̼�Ͷ�������ʱ�õ�����Һ�о�����ˮ�������ȼ���ˮ�Ĵ��ڣ�������̼�Ͷ������������ʹ����ʯ��ˮ����ǣ������ȼ�����������ٳ�ȥ�������������̼����������˳���ǣ��ܡ��ڡ��١���
���ʴ�Ϊ���ܡ��ڡ��١��ۣ�
��2��AΪ�����������װ�ã�B��CΪ��ȥ�����������Ƿ�������������װ�ã�
�ʴ�Ϊ������SO2���壬����SO2���壬����SO2�����Ƿ������
��3����ˮ����ͭΪ��ɫ���壬��ˮ�ɱ�Ϊ��ˮ����ͭ��CuSO4?5H2O��Ϊ��ɫ���壬���ڼ���ˮ�Ĵ��ڣ����ڲ�������ͨ���١���ʱ�����ˮ���������Ԣڱ����ڢ١���֮ǰ��
�ʴ�Ϊ����ˮCuSO4��ˮ���������ڲ�������ͨ���١���ʱ�����ˮ���������Ԣڱ����ڢ١���֮ǰ��
������I��ʵ�������������ƺ��Ȼ����ȡ���������ݰ��������ʼ��ܶ�ȷ���ռ�������
II����1�����������̼�Ͷ�������ʱ�õ�����Һ�о�����ˮ�������ȼ���ˮ�Ĵ��ڣ�������̼�Ͷ������������ʹ����ʯ��ˮ����ǣ������ȼ�����������ٳ�ȥ�������������̼��
��2������ʵ��Ŀ�ĺ�װ��ͼ���Dz���������Լ������ü�������Ӧ�������忼�ǣ�AΪ�����������װ�ã�B��CΪ��ȥ�����������Ƿ�������������װ�ã�
��3������ʵ��Ŀ��֪����Ҫ����ˮ������ˮ������ʹ��ˮ����ͭ���������������������ȷ����ˮ����ͭ��λ�ã�
���������⿼�鰱������ȡ�����ʵļ��飬ע�����������õ��Լ��Լ�������Ⱥ�˳��Ϊ�״��㣬�ѶȽϴ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��ͼ�ش��������⣨���������ѱ������ڵ��������߲����������Ը�����Ҫ���ӣ�
��ͼ�ش��������⣨���������ѱ������ڵ��������߲����������Ը�����Ҫ���ӣ� ��ͼ�ش��������⣨���������ѱ������ڵ��������߲����������Ը�����Ҫ���ӣ�
��ͼ�ش��������⣨���������ѱ������ڵ��������߲����������Ը�����Ҫ���ӣ�