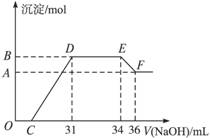
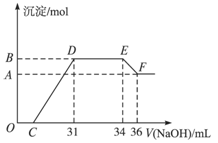
题目内容
某同学取一定量的Al和Fe固体混合物,与2.0 L极稀的硝酸充分反应,反应过程中无气体放出。在反应结束后的溶液中,逐滴加入4 mol/L的氢氧化钠溶液,所加氢氧化钠溶液的体积(mL)与产生的沉淀的物质的量(mol)的关系如图所示。试回答下列问题: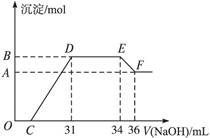
1、图中OC段没有沉淀生成,此阶段发生反应的离子方程式为 。
在DE段,沉淀的物质的量没有变化,则此阶段发生反应的离子方程式为 ;上述现象说明溶液中______________结合OH-的能力比___________强(填写离子符号)。
2、B与A的差值为______________mol。
3、B点对应的沉淀的物质的量为____________mol,C点对应的氢氧化钠溶液的体积为______________mL。
4、求原硝酸溶液的物质的量浓度?
1、H+ + OH—= H2O (1分)、 NH4+ + OH—= NH3·H2O (1分)
Al3+、Fe3+(2分) 、 NH4+ (2分)
2、0.008 (2分)
3、 0.032 (2分)、 7 (2分)
4、0.074 (4分)
解析

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
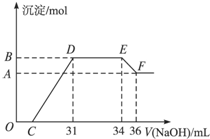 (2012?上海模拟)某同学取一定量的Al和Fe固体混合物,与2.0L极稀的硝酸充分反应,反应过程中无气体放出.在反应结束后的溶液中,逐滴加入4mol/L的氢氧化钠溶液,所加氢氧化钠溶液的体积(mL)与产生的沉淀的物质的量(mol)的关系如图所示.试回答下列问题:
(2012?上海模拟)某同学取一定量的Al和Fe固体混合物,与2.0L极稀的硝酸充分反应,反应过程中无气体放出.在反应结束后的溶液中,逐滴加入4mol/L的氢氧化钠溶液,所加氢氧化钠溶液的体积(mL)与产生的沉淀的物质的量(mol)的关系如图所示.试回答下列问题: