��Ŀ����
�Ƕ���ֽ⵰���ʵĹ��̿ɱ�ʾΪ��������
�����
��
�ֶ�ţ���еĵ����ʽ�������ʵ�飺ȡ30.0mLţ�̣��øǴ�����ֽ⵰���ʣ��ѵ���ȫת��Ϊ������0.500mol?L-1��H2SO4��Һ50.0mL���պ�ʣ�������1.00mol?L-1�� NaOH��Һ�кͣ���38.0mL����
��1��30.0mLţ���е�Ԫ�ص������Ƕ��٣�
��2����֪ţ�̵��ܶ�1.03g?mL-1���������к�������������Ϊ16.0%�����ţ���е����ʵ���������Ϊ���٣�
| ŨH2SO4��Һ |
| ŨNaOH��Һ |
�ֶ�ţ���еĵ����ʽ�������ʵ�飺ȡ30.0mLţ�̣��øǴ�����ֽ⵰���ʣ��ѵ���ȫת��Ϊ������0.500mol?L-1��H2SO4��Һ50.0mL���պ�ʣ�������1.00mol?L-1�� NaOH��Һ�кͣ���38.0mL����
��1��30.0mLţ���е�Ԫ�ص������Ƕ��٣�
��2����֪ţ�̵��ܶ�1.03g?mL-1���������к�������������Ϊ16.0%�����ţ���е����ʵ���������Ϊ���٣�
��1����38mL1.00mol?L-1�� NaOH��Һ�к���������ʵ���Ϊn����
2NaOH+H2SO4=Na2SO4+2H2O
2 1
0.038L��1mol/L n
��ã�n=0.038L��1mol/L��
=0.019mol
�����հ�������������ʵ���Ϊ��0.500mol?L-1��0.05mL-0.019mol=0.006mol
��30.0mLţ���е�Ԫ�ص�������m����
2N��2NH3��H2SO4
28g 1mol
m 0.006mol
����m=28g��
=0.168g
��30.0mLţ���е�Ԫ�ص�������0.168g��
��2��30mLţ�̵�����Ϊ��30mL��1.03g?mL-1=30.9g
����30.9g���أ������ʣ���16.0%=0.168g
��ã��أ������ʣ�=3.4%
�𣺸�ţ���е����ʵ���������Ϊ3.4%��
2NaOH+H2SO4=Na2SO4+2H2O
2 1
0.038L��1mol/L n
��ã�n=0.038L��1mol/L��
| 1 |
| 2 |
�����հ�������������ʵ���Ϊ��0.500mol?L-1��0.05mL-0.019mol=0.006mol
��30.0mLţ���е�Ԫ�ص�������m����
2N��2NH3��H2SO4
28g 1mol
m 0.006mol
����m=28g��
| 0.006mol |
| 1mol |
��30.0mLţ���е�Ԫ�ص�������0.168g��
��2��30mLţ�̵�����Ϊ��30mL��1.03g?mL-1=30.9g
����30.9g���أ������ʣ���16.0%=0.168g
��ã��أ������ʣ�=3.4%
�𣺸�ţ���е����ʵ���������Ϊ3.4%��

��ϰ��ϵ�д�
 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
�����Ŀ
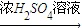 �����
�����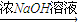 ��
��