��Ŀ����
ijǿ����ҺX�п��ܺ���NH4+��Fe2+��Al3+��CO32-��SO42-��Cl-��NO3-�е������֣�ij�о���ѧϰС��Ϊ̽����ҺX����ɣ�����������ʵ�飨����YΪ��ɫ��ζ�����壩��
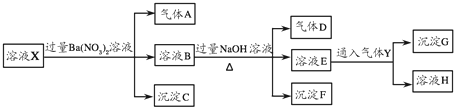
�ش��������⣺
��1����ҺX�п϶����ڵ������� �϶������ڵ������� ��
��2��д����������A�����ӷ���ʽ ��
��3��д������ҺE��ͨ������Yʱһ�����������ӷ���ʽ ��
��4���������ʵ�鷽����һ��ȷ�����ܴ��ڵ����ӣ�д��ʵ��IJ������衢��������ۣ� ��

�ش��������⣺
��1����ҺX�п϶����ڵ�������
��2��д����������A�����ӷ���ʽ
��3��д������ҺE��ͨ������Yʱһ�����������ӷ���ʽ
��4���������ʵ�鷽����һ��ȷ�����ܴ��ڵ����ӣ�д��ʵ��IJ������衢��������ۣ�
���㣺���������ӵļ���,���������ӵļ���
ר�⣺
��������ǿ������Һ��һ���������CO32-����ҺX�м���������ᱵ���ɳ�������ó���ΪBaSO4������˵����Һ�к���SO42-���ӣ���������A��AӦΪNO��˵����Һ�к��л�ԭ�����ӣ�һ��ΪFe2+���ӣ�����Һ�в��ܴ���NO3-����ҺB�м������NaOH��Һ����������D����DΪNH3��˵����Һ�к���NH4+���ӣ�����FΪFe��OH��3����ҺE��ͨ��Y��CO2���壩���ɳ���G����G��һ����̼�ᱵ�����ܺ�Al��OH��3����E����ΪNaAlO2����ҺHΪNaHCO3������˵����Һ�к���������ΪNH4+��Fe2+��һ����������ΪSO42-��һ������CO32-��NO3-���ӣ�����ȷ���Ƿ���Al3+��Cl-���Դ������
���
�⣺��ǿ������Һ��һ���������CO32-����ҺX�м���������ᱵ���ɳ�������ó���ΪBaSO4������˵����Һ�к���SO42-���ӣ���������A��AӦΪNO��˵����Һ�к��л�ԭ�����ӣ�һ��ΪFe2+���ӣ�����Һ�в��ܴ���NO3-����ҺB�м������NaOH��Һ����������D����DΪNH3��˵����Һ�к���NH4+���ӣ�����FΪFe��OH��3����ҺE��ͨ��Y��CO2���壩���ɳ���G����G��һ����̼�ᱵ�����ܺ�Al��OH��3����E����ΪNaAlO2����ҺHΪNaHCO3������˵����Һ�к���������ΪNH4+��Fe2+��һ����������ΪSO42-��һ������CO32-��NO3-���ӣ�����ȷ���Ƿ���Al3+��Cl-��
��1��������������֪��X�к�����ΪNH4+��Fe2+��SO42-��һ������CO32-��NO3-���ӣ��ʴ�Ϊ��NH4+��Fe2+��SO42-��CO32-��NO3-��
��2����������A�����ӷ���ʽΪ3Fe2++4H++NO3-�T3Fe3++NO��+2H2O���ʴ�Ϊ��3Fe2++4H++NO3-�T3Fe3++NO��+2H2O��
��3������ҺE��ͨ������Yʱһ�����������ӷ���ʽBa2++2OH-+CO2�TBaCO3��+H2O���ʴ�Ϊ��Ba2++2OH-+CO2�TBaCO3��+H2O��
��4��������������֪����Һ�п��ܴ��ڵ�����ΪCl-��Al3+��ȡ����G�������������������Һ���������в����ܽ⣬��֤��һ�����������ӣ��ʴ�Ϊ��ȡ����G�������������������Һ���������в����ܽ⣬��֤��һ�����������ӣ�
��1��������������֪��X�к�����ΪNH4+��Fe2+��SO42-��һ������CO32-��NO3-���ӣ��ʴ�Ϊ��NH4+��Fe2+��SO42-��CO32-��NO3-��
��2����������A�����ӷ���ʽΪ3Fe2++4H++NO3-�T3Fe3++NO��+2H2O���ʴ�Ϊ��3Fe2++4H++NO3-�T3Fe3++NO��+2H2O��
��3������ҺE��ͨ������Yʱһ�����������ӷ���ʽBa2++2OH-+CO2�TBaCO3��+H2O���ʴ�Ϊ��Ba2++2OH-+CO2�TBaCO3��+H2O��
��4��������������֪����Һ�п��ܴ��ڵ�����ΪCl-��Al3+��ȡ����G�������������������Һ���������в����ܽ⣬��֤��һ�����������ӣ��ʴ�Ϊ��ȡ����G�������������������Һ���������в����ܽ⣬��֤��һ�����������ӣ�
���������⿼�����ӵ��ƶϣ�Ϊ��Ƶ���㣬���������ķ�����ȷ���������Ӵ��ڣ���ô����֤��һ�������Ӳ����ڣ�

��ϰ��ϵ�д�
 ����5��2���ϵ�д�
����5��2���ϵ�д�
�����Ŀ
��1-������ȡCH3COCOOCH2CH2CH3�����������Ҫ�����з�Ӧ��˳���ǣ�������
a������ b����ԭ c��ȡ�� d���ӳ� e����ȥ f���к� g������ h��������
a������ b����ԭ c��ȡ�� d���ӳ� e����ȥ f���к� g������ h��������
| A��e��d��c��a��h |
| B��b��d��f��g��h |
| C��a��e��d��c��h |
| D��b��a��e��c��f |
ij����С���ͬѧ��ij�ؿ����ɼ����л������������Ʒ��Ȼ��������ˮ��ȡ����̽���������������Գɷֵ�ʵ���У����и���ʵ������ó��Ľ��۲���ȷ���ǣ�������
| A�����ȡҺ�е���AgNO3��Һ�а�ɫ����������˵��������һ������Cl- |
| B�����ȡҺ�е��������ữ��BaCl2��Һ���а�ɫ����������˵��������һ����SO42- |
| C�����ȡҺ�м���Cu��ŨH2SO4���Թܿ��к���ɫ���������˵�������п��ܺ���NO3- |
| D�����ȡҺ�м���ŨNaOH��Һ�ȣ��Թܿڸ���ʪ��ĺ�ɫʯ����ֽ������˵��������һ������NH4+ |
���������м���ʹ���Ը��������Һ����ʹ������Ȼ�̼��Һ��ɫ���ǣ�������
| A���ױ� | B������ϩ |
| C�������� | D���۱���ϩ |
����ʵ������У���ȷ���ǣ�������
A�� ��ˮ���ռ�NO |
B�� ϡ��Ũ���� |
C�� ʵ�����ư��� |
D�� ���� |
��ֲ���з�����Ļ��Ի�����zeylastral�Ľṹ��ʽ��ͼ��ʾ�� ����˵������ȷ���ǣ�������
����˵������ȷ���ǣ�������
 ����˵������ȷ���ǣ�������
����˵������ȷ���ǣ�������| A�������к���6������̼ԭ�� |
| B������FeCl3��Һ��������Һ������Ӧ |
| C��1mol zeylastral�����5molH2������Ӧ |
| D��1mol zeylastral������lmolBr2������Ӧ |
���������ڸ��������¿���ֱ��ת�����ǣ�������
A��Al2O3
| |||||
B��Fe
| |||||
C��SiO2
| |||||
D��CaCl2
|
 ��ӦA+B��C�������� �У���A+B��X����X��C����Ӧ�����������仯��ͼ��ʾ��E1��ʾ��ӦA+B��X�Ļ�ܣ������� ��������ȷ���ǣ�������
��ӦA+B��C�������� �У���A+B��X����X��C����Ӧ�����������仯��ͼ��ʾ��E1��ʾ��ӦA+B��X�Ļ�ܣ������� ��������ȷ���ǣ�������| A��E2��ʾ��ӦX��C�Ļ�� |
| B��X�Ƿ�ӦAʮB��C�Ĵ��� |
| C����ӦAʮB��C�ġ�H��0 |
| D����������ɸı䷴ӦAʮB��C���ʱ� |