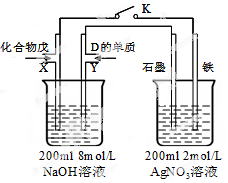
��Ŀ����
����ʵ�顰�����������롰���ۡ���Ӧ��ϵ��ȷ����
| | ���������� | ���� |
| A | ��NaCl��Һ�ȵμ�����AgNO3��Һ����μ�����NaI��Һ�����а�ɫ���������ɻ�ɫ���� | ˵��Ksp(AgI)<Ksp(AgCl) |
| B | ��SO2ͨ��Ʒ����Һ�У���ɫ��ȥ | ˵��SO2����Ư���� |
| C | ��FeCl3��CuCl2�����Һ�����ۣ��к�ɫ�������� | ˵�������ԣ�Cu2+>Fe3+ |
| D | ��ij��Һ�ȵμ������ữ���ٵμ�BaCl2��Һ���а�ɫ�������� | ����Һ��һ������Ag+ |
AB
�������������A��NaCl�м�����AgNO3���ɰ�ɫ����Ϊ��AgCl���ټ�����NaI����ɫ������Ϊ��ɫ��AgI,�ܹ�˵��Ksp(AgI)<Ksp(AgCl)����ȷ��B����Ʒ����Һ����SO2��Ư���ԣ���ȷ��C���������۹������������ԣ�Cu2+>Fe3+���ȷ���2Fe3++Fe=3Fe2+���ٷ�����Cu2++Fe=Fe2++Cu,����D����ʱ��ɫ��������Ϊ��BaSO4��Ӧ�ټ�AgNO3,����
���㣺����ʵ������������۵������жϣ��漰����ת����SO2Ư���ԡ�Fe3+��Cu2+������ǿ���Ƚϡ�Ag+�ļ��顣

��ϰ��ϵ�д�
�����Ŀ
�������ʵ�ת���ڸ�����������ʵ�ֵ���
| A���٢ۢ� | B���ڢۢ� | C���ڢܢ� | D���٢ܢ� |
�����й����ʵ�������Ӧ�����Ӧ����
| A�������������ԣ������ڵ�̲��� |
| B��Na2O2����ǿ�����ԣ�������������ߵĹ����� |
| C���Ȼ�����Һ������ͭ������������ӡˢ��·�� |
| D��SO2����Ư���ԣ���ʹ���Ը��������Һ��ɫ |
�������ʵ�ת���ڸ��������²���ʵ�ֵ���
| A���٢ڢ� | B���ڢۢ� | C���ڢۢ� | D���٢ܢ� |
����ʵ������У�ʼ���������������
| A��O2ͨ���ữ��KI������Һ�� |
| B��CO2ͨ��CaCl2��Һ�� |
| C��0.1mol ? L��1ϡ H2SO4����0.1 mol ? L��1Na2S2O3��Һ�� |
| D��SO2ͨ�����ữ��Ba(NO3)2��Һ�� |
��������ת���ڸ�����������ʵ�ֵ���
��FeS2  SO3
SO3 H2SO4
H2SO4 CO2
CO2
�� Al2O3 NaAlO2(aq)
NaAlO2(aq) Al(OH)3
Al(OH)3
��NaCl(aq) Na
Na NaOH(aq)
NaOH(aq)
��Fe FeSO4(aq)
FeSO4(aq) Fe(OH)2
Fe(OH)2 Fe2O3
Fe2O3
��
| A���٢ۢ� | B���ڢۢ� | C���ڢܢ� | D���٢ܢ� |
���и���ʵ��������������ó��Ľ�����ȷ����
| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | ȡ�����ʵ��������ֽ�������X��Y���ֱ������������ᷴӦ | X���������������Y�� | �����ԣ�X>Y |
| B | ����м��ȵ�AgNO3��Һ�еμ�KCl��Һ | ��Һ�ɺ�ɫ��Ϊ��ɫ | KCl��Һ���м��� |
| C | ��CuSO4��Һ�м���KI��Һ���ټ��뱽���� | �а�ɫ�������ɣ��������ɫ | ��ɫ��������ΪCuI |
| D | ȡ���õ�Na2O2��ĩ�������еμӹ��������� | ������ɫ���� | Na2O2û�б��� |
�±��У��Գ���I�������ȷ�Լ������Ƿ���������ϵ���ж϶���ȷ����
| ѡ�� | ����I | ������ | �ж� |
| A | KSCN��Һ�ɼ���Fe3+ | ����Һ�Ⱥ����������ˮ�ͼ���KSCN��Һ���ΪѪ��ɫ��֤������Һ��Fe3+ | I�ԣ���ԣ��� |
| B | SO2���л�ԭ�� | SO2ͨ��Ba(NO3)2��Һ�пɼ���ɫ�������� | I�ԣ���ԣ��� |
| C | NO2�����������ʺ���ɫ | ��ˮ�ɼ���NO2�������� | I�ԣ�������� |
| D | ��Ӧ�������ͬ�ɵ��²��ﲻͬ | Na��O2��Ӧ��������Na2O��Ҳ��������Na2O2 | I�ԣ���ԣ��� |