��Ŀ����
19����1����֪������HCOOH�ĵ��볣��Ϊ${K_a}=2��{10^{-4}}$����HCOO-��ˮ�ⷴӦHCOO-+H2O?HCOOH+OH-��ƽ�ⳣ��ΪKh=5��10-11����2�������£���a mol/L��HCOOH��Һ��$\frac{a}{2}$mol/L��NaOH��Һ�������Ϻ���Һ�и�����Ũ���ɴ�С��˳����c��HCOO-����c��Na+����c��H+����c��OH-����
��3�������£���a mol/L��HCOOH��Һ�м���������bmol/L��NaOH��Һ����Һ�����ԣ���ʱ��Һ��HCOOH�����ʵ���Ũ�� $\frac{a-b}{2}$��
���� ��1��д���������ƽ�ⳣ�������������ˮ��ƽ�ⳣ����ˮ�����ӻ��������бȽϣ��Ӷ��ó���ϵʽ�����м��㼴�ɣ�
��2����amol/L��HCOOH��Һ��$\frac{a}{2}$mol/L��NaOH��Һ�������Ϻõ����Ǽ���ͼ����ƵĻ����ݴ˻ش��жϣ�
��3��������Һ�еĵ���غ��Լ������غ�֪ʶ�����㣮
��� �⣺��1��Ka=$\frac{c��C{H}_{3}CO{O}^{-}��•c��{H}^{+}��}{c��C{H}_{3}COOH��}$��Kh=$\frac{c��C{H}_{3}COOH��•c��O{H}^{-}��}{c��C{H}_{3}CO{O}^{-}��}$��Kw=C��H+��•C��OH-��������Ka•Kh=Kw��Kh=$\frac{1{0}^{-14}}{2��1{0}^{-4}}$=5��10-11��
�ʴ�Ϊ��5��10-11��
��2��amol/L��HCOOH��Һ��$\frac{a}{2}$mol/L��NaOH��Һ�������Ϻõ����ǵ�Ũ�ȵļ����ƺͼ���Ļ�����Һ��ʾ���ԣ�����ĵ���̶ȴ��ڼ�������ӵ�ˮ��̶ȣ�����Ũ�ȴ�С˳���ǣ�c��HCOO-����c��Na+����c��H+����c��OH-������
�ʴ�Ϊ��c��HCOO-����c��Na+����c��H+����c��OH-������
��3��������Һ��ʾ���ԣ�����c��OH-��=c��H+�������ݵ���غ㣬�õ�c��HCOO-��=c��Na+��=$\frac{b}{2}$mol/L�����������غ㣬��Һ��c��HCOOH��=$\frac{a}{2}$-c��HCOO-��=$\frac{a-b}{2}$mol/L��
�ʴ�Ϊ��$\frac{a-b}{2}$mol/L��
���� ���⿼���˵���ƽ�ⳣ���������ƽ��Ӱ�����غͼ��������Ӧ�á���Һ������Ũ�ȴ�С�Ƚϵ�֪ʶ�����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�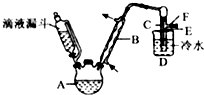 ±���������������о��й㷺��Ӧ�ã��ش��������⣺
±���������������о��й㷺��Ӧ�ã��ش��������⣺��1�����ȴ����鳣Ϊ�л��ܼ�������ӽṹΪ�������������Ϊ���ȼ��飻̼ԭ�Ӹ���������10�����������У���һ�ȴ���ֻ��һ�ֵ������ĸ���Ϊ4����
��2�������������飨CF3CHClBr����һ����������д��������ͬ���칹��ĽṹʽCHFClCF2Br��CHFBrCF2Cl��CFClBrCHF2�������������칹����
��3��������ϩ�������г��õ����ϣ���ҵ����������ϩ��һ�ֹ���·�����£�
��ϩ$��_{��}^{Cl_{2}}$1��2-��������$��_{��}^{480-530��}$����ϩ$\stackrel{�ۺ�}{��}$������ϩ
��Ӧ�ٵķ�Ӧ����Ϊ�ӳɷ�Ӧ��
��Ӧ�ڵķ�Ӧ����Ϊ��ȥ��Һ��
��4��ʵ�������Ҵ���Ũ������廯��Ϊ�Լ�������ͼ��װ���Ʊ������飬ͼ��ʡȥ�˼���װ�ã�
�й����ݼ�����
| �Ҵ� | ������ | �� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | �����ɫҺ�� |
| �ܶȣ�g/cm3�� | 0.79 | 1.44 | 3.1 |
| �е� | 78.5 | 38.4 | 59 |
���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ����AB������ţ�
A�����ٸ�����ϩ���ѵ����� B������Br2������ C��ˮ�Ƿ�Ӧ�Ĵ��� D�������Ũ��Խϡ��ӦԽ��
�ۼ���Ӧ��ȡ�ķ�ʽΪˮԡ���ȣ����ȵ�Ŀ���������¶ȣ��ӿ췴Ӧ���ʣ�ͬʱʹ���ɵ��������������ٽ�ƽ�����ƣ�
��Ϊ��ȥ�ռ���Ʒ�е���Ҫ���ʣ�Ӧѡȡ����ĺ����Լ�ΪNa2SO3��Һ��NaHSO3��Һ�����õIJ����Ƿ�Һ��
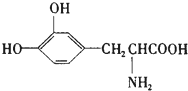 L-�����һ���л��������������ɭ�ۺ�֢�����ƣ���ṹ��ʽ��ͼ������ҩ��������ǻ��ڻ��2000��ŵ��������ѧ��ҽѧ����2001��ŵ������ѧ�����о��ɹ������й���L-��͵�������ȷ���ǣ�������
L-�����һ���л��������������ɭ�ۺ�֢�����ƣ���ṹ��ʽ��ͼ������ҩ��������ǻ��ڻ��2000��ŵ��������ѧ��ҽѧ����2001��ŵ������ѧ�����о��ɹ������й���L-��͵�������ȷ���ǣ�������| A�� | ֻ����Ӧ���������ᷴӦ | |
| B�� | 1mol�����������4molNaOH��Ӧ | |
| C�� | �����ʲ���ʹ����KMnO4��ɫ | |
| D�� | 1mol������������1.5molHBr��Ӧ |
| A�� | CH��CH�� | B�� | ��ϩ�ͻ����� | C�� | 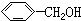 �� ��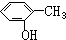 | D�� | ���Ѻͼ״� |
 ��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ���ش��������⣺
��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ���ش��������⣺| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | Ka=1.8��10-5 | Ka1=4.3��10-7 Ka2=5.6��10-11 | Ka=3.0��10-8 |
a��CH3COONa��������������b��Na2CO3 c��NaClO d��NaHCO3
pH��С�������е�˳����a��d��c��b���ñ����д����
��2�������£�0.1mol•L-1 CH3COOH��Һ��ˮϡ�����У����б���ʽ�����ݱ�����BD������ĸ����
A��c��H+�� B.$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ C��c��H+��•c��OH-�� D.$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$ E.$\frac{c��{H}^{+}��•c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$
��3��д�������������Һ��ͨ������������̼�����ӷ���ʽ��ClO-+H2O+CO2=HCO3-+HClO��
��4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH=6������Һ��c��CH3COO-��-c��Na+��=9.9��10-7mol•L-1����ȷ��ֵ����
��5��25��ʱ����a mol•L-1�Ĵ�����b mol•L-1�������Ƶ������ϣ���Ӧ����Һǡ�������ԣ���a��b��ʾ����ĵ���ƽ�ⳣ��Ϊ$\frac{b��10-7}{a-b}$��
��6�������Ϊ100mL pH=2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ����HX�ĵ���ƽ�ⳣ�����ڣ�����ڡ�����С�ڡ����ڡ���CH3COOH�ĵ���ƽ�ⳣ����
��7����״���£���1.12L CO2ͨ��100mL 1mol•L-1��NaOH��Һ�У�����Һ������Ũ�ȷ���������е�ʽ��
��c��OH-��=2c��H2CO3��+c��HCO3-��+c��H+����
��c��H+��+c��Na+��=2c��CO32-��+c��HCO3-��+c��OH-����
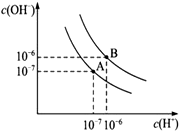 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ�� �ĺϳ�·�ߣ��������Լ���ѡ������֪
�ĺϳ�·�ߣ��������Լ���ѡ������֪ ��
��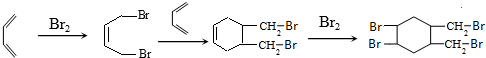 ��
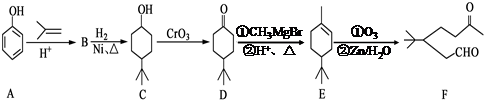
��
 ��
�� ��ͬʱ��������������B��ͬ���칹�壨������B������11�֣�
��ͬʱ��������������B��ͬ���칹�壨������B������11�֣�