��Ŀ����
����Ŀ��50 mL 0.50 mol��L1������50 mL 0.55 mol��L1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
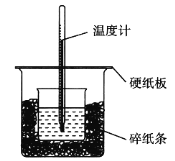
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��______________________________��
��2���ձ���������ֽ����������______________��
��3�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ__________(����ƫ������ƫС��������Ӱ����)��
��4��ʵ���и���60 mL 0.50 mol��L1�����50 mL 0.55 mol��L1 NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������__________(��������������������)�������к���__________(��������������������)���������ɣ�_________________________��
��5������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��__________(����ƫ������ƫС��������Ӱ��������ͬ)����50 mL 0.50 mol��L1 NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��__________��
���𰸡����β�������� ����ʵ������е�������ʧ ƫС ����� ��� ��Ϊ�к�����ָ�������кͷ�Ӧ����1 mol H2O(l)���ų��������������������� ƫС ƫС
��������
��ʵ��Ĺؼ�Ϊȷ�ⶨ��Ӧ����¶ȣ������㷴Ӧ����1molˮʱ�ķ�Ӧ�ȼ��к��ȡ�����ʵ���б��¾����ǹؼ���ȷ�ⶨʵ������Ҳ�ܹؼ�����֤������ȫ��Ӧ�����������к��ȡ�
��1����ʵ��ɰܵĹؼ���ȷ������Ӧ����¶ȡ��������װ�ñ��뱣�¡������ҿ�ʹ��ϵ�¶Ⱦ���ﵽһ�£���ȱ�ٵ�����ӦΪ���β����������
��2����ֽ��������Ϊ����ʵ������е�������ʧ��
��3������Ӳֽ�����ʧ������������������ƫС��
��4�����к��ȵĸ����֪���к�����������1 molˮΪ���ģ���������ֵ�����أ����Էų�����������ȣ����к�����ȡ�
��5���������ᡢ������кͷ�Ӧ�ų�������ͬʱ���������ᡢ����ĵ������ȣ������ð�ˮ����NaOH����õ��к�����ֵƫС����50 mL 0.50 mol��L1 NaOH��Һ��������ʵ��ᵼ�·�Ӧ����֣���õķ�Ӧ�Ȼ�ƫС��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ԭCO2�ǽ������ЧӦ����Դ�������Ҫ�ֶ�֮һ���о���������Cu/ZnO���������£�CO2��H2�ɷ�������ƽ�з�Ӧ���ֱ�����CH3OH��CO����Ӧ���Ȼ�ѧ����ʽ���£�
CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H1=-53.7kJ��mol-1 I
CH3OH(g)+H2O(g) ��H1=-53.7kJ��mol-1 I
CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H2 II
CO(g)+H2O(g) ��H2 II
ijʵ���ҿ���CO2��H2��ʼͶ�ϱ�Ϊ1��2.2������ͬѹǿ�£�������ͬ��Ӧʱ��������ʵ�����ݣ�
T(K) | ���� | CO2ת���ʣ�%�� | �״�ѡ���ԣ�%�� |
543 | Cat.1 | 12.3 | 42.3 |
543 | Cat.2 | 10.9 | 72.7 |
553 | Cat.1 | 15.3 | 39.1 |
553 | Cat.2 | 12.0 | 71.6 |
����ע��Cat.1��Cu/ZnO���װ���Cat.2��Cu/ZnO����Ƭ���״�ѡ���ԣ�ת����CO2�����ɼ״��İٷֱ�
��֪����CO��H2�ı�ȼ���ȷֱ�Ϊ-283.0kJ��mol-1��-285.8kJ��mol-1
��H2O(l)=H2O(g) ��H3=44.0kJ��mol-1
��ش𣨲������¶ȶ���H��Ӱ�죩��
(1)��ӦI��ƽ�ⳣ������ʽK=___��
(2)���������CO2ת��ΪCH3OHƽ��ת���ʵĴ�ʩ��___��
A��ʹ�ô���Cat.1
B��ʹ�ô���Cat.2
C�����ͷ�Ӧ�¶�
D��Ͷ�ϱȲ��䣬���ӷ�Ӧ���Ũ��
E������CO2��H2�ij�ʼͶ�ϱ�
(3)����ʵ�����ݱ���������ͬ�¶��²�ͬ�Ĵ�����CO2ת����CH3OH��ѡ������������Ӱ�죬��ԭ����___��
(4)��ͼ�зֱ���ӦI����������Cat.1����Cat.2��������¡���Ӧ���̡�������ʾ��ͼ___��