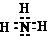��Ŀ����
����Դ��һ����Ҫ�����Դ���������ֿɲ���H2�Ļ�������ң���6.00g��������ȫ�ֽ⣬ֻ�õ�һ�ֶ�����Ԫ�صĽ������ʺ�6.72L��H2��������ɱ�״����������ˮ��ӦҲ�ܷų�H2��ͬʱ������һ�ְ�ɫ������ð�ɫ����������NaOH��Һ�����������ڴ��������¿ɷֽ�õ�H2����һ�ֵ�������������ڱ�״���µ��ܶ�Ϊ1.25g?L-1����ش��������⣺
��1���Ļ�ѧʽ��______���ҵĵ���ʽ��______��
��2������ˮ��Ӧ�Ļ�ѧ����ʽ��______��
��3������������þ��Ӧ�IJ�����______���û�ѧʽ��ʾ����
��4�����ڼ�����������CuO��Ӧ������Cu���������д���÷�Ӧ�Ļ�ѧ����ʽ______�������������Cu�п��ܻ�����Cu2O�������ʵ�鷽����֤֮______������֪��Cu2O+2H+�TCu+Cu2++H2O��
��5��������֮��______������ܡ������ܡ���������Ӧ����H2���ж�������______��
�⣺��ɫ����������NaOH��Һ��ӦΪAl��OH��3��˵�����к���Al��H����Ԫ�أ�n��H2��= =0.3mol��
=0.3mol��
��m��H��=0.3mol��2��1g/mol=0.6g����6.00g�����m9Al��=6.00g-0.6g=5.4g��n��Al��= =0.2mol��
=0.2mol��
����n��Al����n��H��=0.2mol��0.6mol=1��3����Ļ�ѧʽΪAlH3�����ڱ�״���µ��ܶ�Ϊ1.25g?L-1����������ԭ������Ϊ1.25g?L-1��22.4L=28��ӦΪN2������ΪNH3��
��1�������Ϸ�����֪��ΪAlH3����ΪNH3������ʽΪ ���ʴ�Ϊ��AlH3��
���ʴ�Ϊ��AlH3�� ��
��
��2��AlH3��ˮ����������ԭ��Ӧ����Ӧ�ķ���ʽΪAlH3+3H2O=Al��OH��3��+3H2����
�ʴ�Ϊ��AlH3+3H2O=Al��OH��3��+3H2����
��3��þ���ڵ�����ȼ�����ɵ���þ����ѧʽΪMg3N2���ʴ�Ϊ��Mg3N2��
��4��NH3�ڼ�����������CuO��Ӧ������Cu������N2����Ӧ�ķ���ʽΪ3CuO+2NH3 N2+3Cu+3H2O��Ҫ�жϲ������Ƿ���CuO���ɼ���ϡ���������Һ�Ƿ������������ȡ�����H2SO4�������Һ������˵�������к���
N2+3Cu+3H2O��Ҫ�жϲ������Ƿ���CuO���ɼ���ϡ���������Һ�Ƿ������������ȡ�����H2SO4�������Һ������˵�������к���
Cu2O����֮����Cu2O��
�ʴ�Ϊ��3CuO+2NH3 N2+3Cu+3H2O��ȡ�����H2SO4�������Һ������˵�������к���Cu2O����֮����
N2+3Cu+3H2O��ȡ�����H2SO4�������Һ������˵�������к���Cu2O����֮����
Cu2O��
��5��AlH3�е�HΪ-1�ۣ�NH3�е�HΪ+1�ۣ��ӻ��ϼ۵ĽǶȿ�֪���߿��ܷ���������ԭ��Ӧ����������
�ʴ�Ϊ�����ܣ�AlH3�е�HΪ-1�ۣ�NH3�е�HΪ+1�ۣ��п��ܷ���������ԭ��Ӧ����������
��������ɫ����������NaOH��Һ��ӦΪAl��OH��3��˵�����к���Al��H����Ԫ�أ�n��H2��= =0.3mol��
=0.3mol��
��m��H��=0.3mol��2��1g/mol=0.6g����6.00g�����m9Al��=6.00g-0.6g=5.4g��n��Al��= =0.2mol��
=0.2mol��
����n��Al����n��H��=0.2mol��0.6mol=1��3����Ļ�ѧʽΪAlH3�����ڱ�״���µ��ܶ�Ϊ1.25g?L-1����������ԭ������Ϊ1.25g?L-1��22.4L=28��ӦΪN2������ΪNH3����϶�Ӧ�������Լ���ĿҪ��ɽ����⣮
���������⿼��������ƶϣ���Ŀ�ѶȲ���ע������ȹط�Ӧ�������Լ������жϼ����������ʣ�ע����ط�Ӧ�Ļ�ѧ����ʽ����д��
 =0.3mol��
=0.3mol����m��H��=0.3mol��2��1g/mol=0.6g����6.00g�����m9Al��=6.00g-0.6g=5.4g��n��Al��=
 =0.2mol��
=0.2mol������n��Al����n��H��=0.2mol��0.6mol=1��3����Ļ�ѧʽΪAlH3�����ڱ�״���µ��ܶ�Ϊ1.25g?L-1����������ԭ������Ϊ1.25g?L-1��22.4L=28��ӦΪN2������ΪNH3��
��1�������Ϸ�����֪��ΪAlH3����ΪNH3������ʽΪ
 ���ʴ�Ϊ��AlH3��
���ʴ�Ϊ��AlH3�� ��
����2��AlH3��ˮ����������ԭ��Ӧ����Ӧ�ķ���ʽΪAlH3+3H2O=Al��OH��3��+3H2����
�ʴ�Ϊ��AlH3+3H2O=Al��OH��3��+3H2����
��3��þ���ڵ�����ȼ�����ɵ���þ����ѧʽΪMg3N2���ʴ�Ϊ��Mg3N2��
��4��NH3�ڼ�����������CuO��Ӧ������Cu������N2����Ӧ�ķ���ʽΪ3CuO+2NH3
 N2+3Cu+3H2O��Ҫ�жϲ������Ƿ���CuO���ɼ���ϡ���������Һ�Ƿ������������ȡ�����H2SO4�������Һ������˵�������к���
N2+3Cu+3H2O��Ҫ�жϲ������Ƿ���CuO���ɼ���ϡ���������Һ�Ƿ������������ȡ�����H2SO4�������Һ������˵�������к���Cu2O����֮����Cu2O��
�ʴ�Ϊ��3CuO+2NH3
 N2+3Cu+3H2O��ȡ�����H2SO4�������Һ������˵�������к���Cu2O����֮����
N2+3Cu+3H2O��ȡ�����H2SO4�������Һ������˵�������к���Cu2O����֮����Cu2O��
��5��AlH3�е�HΪ-1�ۣ�NH3�е�HΪ+1�ۣ��ӻ��ϼ۵ĽǶȿ�֪���߿��ܷ���������ԭ��Ӧ����������
�ʴ�Ϊ�����ܣ�AlH3�е�HΪ-1�ۣ�NH3�е�HΪ+1�ۣ��п��ܷ���������ԭ��Ӧ����������
��������ɫ����������NaOH��Һ��ӦΪAl��OH��3��˵�����к���Al��H����Ԫ�أ�n��H2��=
 =0.3mol��
=0.3mol����m��H��=0.3mol��2��1g/mol=0.6g����6.00g�����m9Al��=6.00g-0.6g=5.4g��n��Al��=
 =0.2mol��
=0.2mol������n��Al����n��H��=0.2mol��0.6mol=1��3����Ļ�ѧʽΪAlH3�����ڱ�״���µ��ܶ�Ϊ1.25g?L-1����������ԭ������Ϊ1.25g?L-1��22.4L=28��ӦΪN2������ΪNH3����϶�Ӧ�������Լ���ĿҪ��ɽ����⣮
���������⿼��������ƶϣ���Ŀ�ѶȲ���ע������ȹط�Ӧ�������Լ������жϼ����������ʣ�ע����ط�Ӧ�Ļ�ѧ����ʽ����д��

��ϰ��ϵ�д�
�����Ŀ