��Ŀ����
����A��B��C��D��E��F����Ԫ�أ�Aԭ�Ӻ���ֻ��һ�����ӣ�B�����������Ǵ�����������2����CԪ�ص����������ӵ�3d�ܼ�Ϊ������� Eԭ�ӵ�M���N��ֱ��Dԭ�ӵ�M���N���5�����ӣ�Fԭ�ӵ�N��ֻ��һ�����ӣ���M��Ϊȫ������
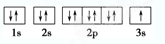
��1��д��B�Ļ�̬ԭ�ӵļ۵��ӹ����ʾʽ
��2��C��Ԫ�ط��� �����̬ԭ�ӵĵ����Ų�ʽΪ
��3����E��C�ܷ�Ӧ�������ӻ�����CE3,��D��ԭ�ӽṹʾ��ͼΪ ��A��B��ԭ�Ӹ�����Ϊ1:1��ɵ���Է�������Ϊ78�ķ�������E�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ
��4����Ԫ��F������������μ��백ˮ��������д���ù����漰��Ӧ�����ӷ���ʽ��
��1��
��2��Fe 1s22s22p63s23p63 d64s2 FeBr3
d64s2 FeBr3
(3) ��  + Br2 ��
+ Br2 �� +HBr
+HBr
(4) Cu2++2NH3?H2O=Cu(OH)2+2NH4+ Cu(OH)2+ 4NH3?H2O=" Cu" (NH3) 42++2OH-+4H2O
����



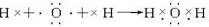



 NH4����OH�������ж�����ˮ���γɵĺ����ṹ��________��(����ͼ�е���ĸ)��
NH4����OH�������ж�����ˮ���γɵĺ����ṹ��________��(����ͼ�е���ĸ)�� 