��Ŀ����
����A��B��C��D���ֶ�����Ԫ�أ����ǵĺ˵������������A��C��B��D�ֱ���ͬ��Ԫ�أ�B��D��Ԫ�ص�������֮����A��C��Ԫ��������֮�͵�2����������Ԫ������һ��Ԫ�صĵ��ʳʵ���ɫ����������ƶϻش���������
��1������Ԫ�ص����Ʒֱ��ǣ�A
��2����д����B����������ߵĻ�����Ļ�ѧʽ��
��3��д��������Ԫ���γɵľ���Ư�����õ��������ʵĻ�ѧʽ��
��4������C���ӽṹʾ��ͼ
 ��д����C��һ�����ӵ�ԭ�ӵĵ���ʽ
��д����C��һ�����ӵ�ԭ�ӵĵ���ʽ

��5���ֱ�д����A��B��Ԫ���γ����ֻ�����Ļ�ѧʽ
��6���ֱ��õ���ʽд��A2B2��C2B2���γɹ���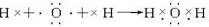
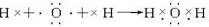 ��
��

��7��д��NH4Cl�ĵ���ʽ��
 ��CO2�ĵ���ʽ��
��CO2�ĵ���ʽ��
 ��
��
��1������Ԫ�ص����Ʒֱ��ǣ�A
��
��
��B��
��
��C��
��
��D��
��
��2����д����B����������ߵĻ�����Ļ�ѧʽ��
H2O2
H2O2
��3��д��������Ԫ���γɵľ���Ư�����õ��������ʵĻ�ѧʽ��
Na2O2
Na2O2
��O3
O3
��H2O2
H2O2
��SO2
SO2
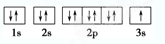
��4������C���ӽṹʾ��ͼ




��5���ֱ�д����A��B��Ԫ���γ����ֻ�����Ļ�ѧʽ
H2O
H2O
��H2O2
H2O2
��6���ֱ��õ���ʽд��A2B2��C2B2���γɹ���
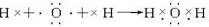
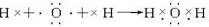


��7��д��NH4Cl�ĵ���ʽ��




��������ɫ�ʵ���ɫ�ĵ���ΪS����Sͬ����Ķ�����Ԫ��ΪO������������֮��Ϊ16+8=24��B��D��Ԫ�ص�������֮����A��C��Ԫ��������֮�͵�2������BΪOԪ�أ�DΪSԪ�أ�A��C������֮��Ϊ12����ͬ���壬��������AΪHԪ�أ�CΪNaԪ�أ����Ԫ�ض�Ӧ���ʡ�������Ľṹ�����ʽ����⣮
����⣺��1����ɫ�ʵ���ɫ�ĵ���ΪS����Sͬ����Ķ�����Ԫ��ΪO������������֮��Ϊ16+8=24��B��D��Ԫ�ص�������֮����A��C��Ԫ��������֮�͵�2������BΪOԪ�أ�DΪSԪ�أ�A��C������֮��Ϊ12����ͬ���壬��������AΪHԪ�أ�CΪNaԪ�أ����Ʒֱ�ΪA���� B���� C���� D���ʴ�Ϊ���⣻�����ƣ���
��2����O����������ߵĻ�����ΪH2O2���ʴ�Ϊ��H2O2��
��3��������Ԫ���γɵľ���Ư�����õ��������ʷֱ���Na2O2��O3��H2O2��SO2���ʴ�Ϊ��Na2O2��O3��H2O2��SO2��
��4��C����ΪNa+��ԭ�Ӻ�����11�����ӣ�ԭ�Ӻ�����10�����ӣ��ṹʾ��ͼΪ ����Na��һ�����ӵ�ԭ��ΪNe������ʽΪ
����Na��һ�����ӵ�ԭ��ΪNe������ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
��5���ֱ�д����H��O��Ԫ���γ����ֻ�����ֱ�ΪH2O��H2O2���ʴ�Ϊ��H2O��H2O2��
��6��H2O2�ĵ���ʽ��ʾ���γɹ���Ϊ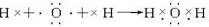 ��Na2O2�ĵ���ʽ��ʾ���γɹ���Ϊ
��Na2O2�ĵ���ʽ��ʾ���γɹ���Ϊ ��
��
�ʴ�Ϊ��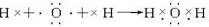 ��
�� ��
��
��7��NH4ClΪ���ӻ��������ʽΪ ��CO2Ϊ���ۻ��������ʽΪ
��CO2Ϊ���ۻ��������ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
��2����O����������ߵĻ�����ΪH2O2���ʴ�Ϊ��H2O2��
��3��������Ԫ���γɵľ���Ư�����õ��������ʷֱ���Na2O2��O3��H2O2��SO2���ʴ�Ϊ��Na2O2��O3��H2O2��SO2��
��4��C����ΪNa+��ԭ�Ӻ�����11�����ӣ�ԭ�Ӻ�����10�����ӣ��ṹʾ��ͼΪ
 ����Na��һ�����ӵ�ԭ��ΪNe������ʽΪ
����Na��һ�����ӵ�ԭ��ΪNe������ʽΪ ��
���ʴ�Ϊ��
 ��
�� ��
����5���ֱ�д����H��O��Ԫ���γ����ֻ�����ֱ�ΪH2O��H2O2���ʴ�Ϊ��H2O��H2O2��
��6��H2O2�ĵ���ʽ��ʾ���γɹ���Ϊ
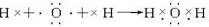 ��Na2O2�ĵ���ʽ��ʾ���γɹ���Ϊ
��Na2O2�ĵ���ʽ��ʾ���γɹ���Ϊ ��
���ʴ�Ϊ��
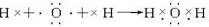 ��
�� ��
����7��NH4ClΪ���ӻ��������ʽΪ
 ��CO2Ϊ���ۻ��������ʽΪ
��CO2Ϊ���ۻ��������ʽΪ ��
���ʴ�Ϊ��
 ��
�� ��
�����������⿼��Ԫ�ص��ƶϣ���Ŀ�Ѷ��еȣ�����϶�����ʽ����д��ѧϰ��ע�����ʽ����д������

��ϰ��ϵ�д�
�����Ŀ
 NH4����OH�������ж�����ˮ���γɵĺ����ṹ��________��(����ͼ�е���ĸ)��
NH4����OH�������ж�����ˮ���γɵĺ����ṹ��________��(����ͼ�е���ĸ)��