��Ŀ����
��98%��Ũ���ᣨ���ܶ�Ϊ1.84g/cm3������100mL1.0mol?L-1ϡ���ᣬ��ʵ�������У�
A��100mL��Ͳ B��������ƽ C�������� D��50mL����ƿ
E��10mL��Ͳ F����ͷ�ι� G��50mL�ձ� H��100mL����ƿ
��1������ȡŨ��������Ϊ mL��
��2��ʵ��ʱѡ�õ������У�����ţ� ��
��3�����ƹ����У����������ʹŨ��ƫ�͵��� ��ƫ�ߵ��� ������ţ���
�ٶ���ʱ���ӿ̶��߹۲�Һ�� ������ƿʹ��ʱδ����
�۶��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ٲ�������ˮ���̶���
������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
����Һע������ƿǰû�лָ������¾ͽ��ж���
����Ͳ�в������Һϴ�Ӻ�Ҳ��������ƿ��
�����ձ����ܽ����ʽ���ʱ������������Һ
�ཫ����õ���Һ������ƿת�Ƶ��Լ�ƿʱ��������������
A��100mL��Ͳ B��������ƽ C�������� D��50mL����ƿ
E��10mL��Ͳ F����ͷ�ι� G��50mL�ձ� H��100mL����ƿ
��1������ȡŨ��������Ϊ
��2��ʵ��ʱѡ�õ������У�����ţ�
��3�����ƹ����У����������ʹŨ��ƫ�͵���
�ٶ���ʱ���ӿ̶��߹۲�Һ�� ������ƿʹ��ʱδ����
�۶��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ٲ�������ˮ���̶���
������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
����Һע������ƿǰû�лָ������¾ͽ��ж���
����Ͳ�в������Һϴ�Ӻ�Ҳ��������ƿ��
�����ձ����ܽ����ʽ���ʱ������������Һ
�ཫ����õ���Һ������ƿת�Ƶ��Լ�ƿʱ��������������
���㣺��Һ������
ר�⣺ʵ����
��������1������c=
�����Ũ��������ʵ���Ũ�ȣ��ٸ�����Һϡ���������ʵ����ʵ�������������ҪŨ����������
��2����������һ�����ʵ���Ũ�ȵ���Һ����ѡ��������Ȼ���ж���Ҫ��������
��3������c=
�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��
| 1000��w |
| M |
��2����������һ�����ʵ���Ũ�ȵ���Һ����ѡ��������Ȼ���ж���Ҫ��������
��3������c=
| n |
| V |
���
�⣺��1��98%��Ũ���ᣨ���ܶ�Ϊ1.84g/cm3�������ʵ���Ũ��Ϊ��c=
mol/L=18.4mol/L������100mL1.0mol?L-1ϡ���ᣬ��ҪŨ��������Ϊ��
��0.0054L=5.4mL��
�ʴ�Ϊ��5.4��
��2������100mL1.0mol?L-1ϡ����IJ���Ϊ�����㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���Ҫ������Ϊ��C����������E��10mL��Ͳ��F����ͷ�ιܡ�G��50mL�ձ���H��100mL����ƿ��
�ʴ�Ϊ��C��E��F��G��H��
��3���ٶ���ʱ���ӿ̶��߹۲�Һ�棬���¼��������ˮ���С������ƿ�̶��ߣ����Ƶ���Һ���ƫС�����Ƶ���ҺŨ��ƫ�ߣ�
������ƿʹ��ʱδ��������ʵ����ʵ�������Һ�����������û��Ӱ�죬���Բ�Ӱ�����ƽ����
�۶��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ٲ�������ˮ���̶��ߣ��������Ƶ���Һ���ƫ����ҺŨ��ƫ�ͣ�
������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬������ȡ��Ũ�������ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
����Һע������ƿǰû�лָ������¾ͽ��ж��ݣ��ȵ���Һ���ƫ����ȴ����Һ�����С���������Ƶ���Һ���ƫС����ҺŨ��ƫ�ߣ�
����Ͳ�в������Һϴ�Ӻ�Ҳ��������ƿ�У��������Ƶ���Һ�����ʵ����ʵ���ƫ�����Ƶ���ҺŨ��ƫ�ߣ�
�����ձ����ܽ����ʽ���ʱ������������Һ���������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
�ཫ����õ���Һ������ƿת�Ƶ��Լ�ƿʱ��������������������Һ�Ǿ�һ���ȶ��ģ������Ƶ���ҺŨ��û��Ӱ�죻
�������Ϸ�����֪�����Ƶ���ҺŨ��ƫ�͵�Ϊ���ۢܢߣ�Ũ��ƫ�ߵ��У��٢ݢޣ�
�ʴ�Ϊ���ۢܢߣ��٢ݢޣ�
| 1000��1.84��98% |
| 98 |
| 1mol/L��0.1L |
| 18.4mol/l |
�ʴ�Ϊ��5.4��
��2������100mL1.0mol?L-1ϡ����IJ���Ϊ�����㡢��ȡ��ϡ�͡���ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���Ҫ������Ϊ��C����������E��10mL��Ͳ��F����ͷ�ιܡ�G��50mL�ձ���H��100mL����ƿ��
�ʴ�Ϊ��C��E��F��G��H��
��3���ٶ���ʱ���ӿ̶��߹۲�Һ�棬���¼��������ˮ���С������ƿ�̶��ߣ����Ƶ���Һ���ƫС�����Ƶ���ҺŨ��ƫ�ߣ�
������ƿʹ��ʱδ��������ʵ����ʵ�������Һ�����������û��Ӱ�죬���Բ�Ӱ�����ƽ����
�۶��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ٲ�������ˮ���̶��ߣ��������Ƶ���Һ���ƫ����ҺŨ��ƫ�ͣ�
������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬������ȡ��Ũ�������ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
����Һע������ƿǰû�лָ������¾ͽ��ж��ݣ��ȵ���Һ���ƫ����ȴ����Һ�����С���������Ƶ���Һ���ƫС����ҺŨ��ƫ�ߣ�
����Ͳ�в������Һϴ�Ӻ�Ҳ��������ƿ�У��������Ƶ���Һ�����ʵ����ʵ���ƫ�����Ƶ���ҺŨ��ƫ�ߣ�
�����ձ����ܽ����ʽ���ʱ������������Һ���������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
�ཫ����õ���Һ������ƿת�Ƶ��Լ�ƿʱ��������������������Һ�Ǿ�һ���ȶ��ģ������Ƶ���ҺŨ��û��Ӱ�죻
�������Ϸ�����֪�����Ƶ���ҺŨ��ƫ�͵�Ϊ���ۢܢߣ�Ũ��ƫ�ߵ��У��٢ݢޣ�
�ʴ�Ϊ���ۢܢߣ��٢ݢޣ�
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ������������е��Ѷȵ����⣬���������ǿ���������У�ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������������ѵ�������������ע����ȷ�������ķ�����

��ϰ��ϵ�д�
 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
�����Ŀ
���й��� Na2CO3 �� NaHCO3 ���ʵıȽ��У���ȷ���ǣ�������
| A�����ȶ��ԣ�Na2CO3��NaHCO3 |
| B����ͬ�����£�����ˮ��Һ�ļ��ԣ�Na2CO3��NaHCO3 |
| C�������£���ˮ�е��ܽ�ȣ�Na2CO3��NaHCO3 |
| D����ͬ�����£���ϡ���ᷴӦ�Ŀ�����Na2CO3��NaHCO3 |
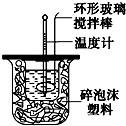 ��ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
��ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£� ͼ��ÿһ�����ʾ�йص�һ�ַ�Ӧ����������֪B��һ�ֵ��ʣ��������ʶ��Ǻ���BԪ�صĻ����C��һ�����Σ�E��C��Ӧ���B��D�ľ��嶼�Ǹ��۵㡢��Ӳ�Ĺ��壬��DΪB���������������BԪ�صķ�Ӧ���Լ�������Ӧ���ӵı�Ҫ�Լ��ͷ�Ӧ����������ȥ����
ͼ��ÿһ�����ʾ�йص�һ�ַ�Ӧ����������֪B��һ�ֵ��ʣ��������ʶ��Ǻ���BԪ�صĻ����C��һ�����Σ�E��C��Ӧ���B��D�ľ��嶼�Ǹ��۵㡢��Ӳ�Ĺ��壬��DΪB���������������BԪ�صķ�Ӧ���Լ�������Ӧ���ӵı�Ҫ�Լ��ͷ�Ӧ����������ȥ����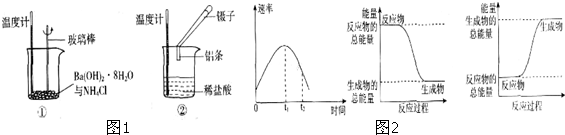
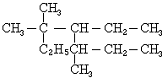 A��ij����2���������ӳɺ�õ�2��2-�������飬��ϵͳ������������������
A��ij����2���������ӳɺ�õ�2��2-�������飬��ϵͳ������������������