��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�һ�������ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ�ش��������⣮
��1���ݡ��ޡ��ߵ�ԭ�Ӱ뾶�ɴ�С��˳��
��2���ڡ��ۡ������ۺ������������ǿ������˳����
��3���١��ܡ��ޡ����е�ijЩԪ�ؿ����γɼȺ������Ӽ��ֺ��й��ۼ��Ļ����д���������ֲ�ͬ������ʵĻ�ѧʽ
��4���ϱ������г��IJ���Ԫ�����γɶ��ִ���18�����ӵķ��ӣ���д���������ֵĻ�ѧʽ

��1���ݡ��ޡ��ߵ�ԭ�Ӱ뾶�ɴ�С��˳��
Na��Al��F
Na��Al��F
����2���ڡ��ۡ������ۺ������������ǿ������˳����
HNO3��H2CO3��H2SiO3
HNO3��H2CO3��H2SiO3
����3���١��ܡ��ޡ����е�ijЩԪ�ؿ����γɼȺ������Ӽ��ֺ��й��ۼ��Ļ����д���������ֲ�ͬ������ʵĻ�ѧʽ
NaOH
NaOH
��NaClO����NaClO3��NaClO4��
NaClO����NaClO3��NaClO4��
����4���ϱ������г��IJ���Ԫ�����γɶ��ִ���18�����ӵķ��ӣ���д���������ֵĻ�ѧʽ
SiH4��HCl��Ar��H2O2��F2��N2H4����д���ּ��ɣ�
SiH4��HCl��Ar��H2O2��F2��N2H4����д���ּ��ɣ�
������������Ԫ�������ڱ��еķֲ���������֪����H������C������N������O������F������Na������Al������Si������Cl������Ar��
��1�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ����Ԫ��ԭ�Ӵ����Ұ뾶��С��
��2��ͬ����Ԫ�ص�����������Ӧˮ������������ǿ��ͬפ������������Ӧˮ��������������
��3���Ⱥ������Ӽ��ֺ��й��ۼ��Ļ�����һ���Ǻ��н��������Ӻ���������ŵ��λ���ǿ�
��4�����ӵĵ����������ڷ����и�ԭ�Ӻ��еĵ������ĺͣ�
��1�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ����Ԫ��ԭ�Ӵ����Ұ뾶��С��
��2��ͬ����Ԫ�ص�����������Ӧˮ������������ǿ��ͬפ������������Ӧˮ��������������
��3���Ⱥ������Ӽ��ֺ��й��ۼ��Ļ�����һ���Ǻ��н��������Ӻ���������ŵ��λ���ǿ�
��4�����ӵĵ����������ڷ����и�ԭ�Ӻ��еĵ������ĺͣ�
����⣺����Ԫ�������ڱ��еķֲ���������֪����H������C������N������O������F������Na������Al������Si������Cl������Ar��
��1�����ݵ��Ӳ�Խ�࣬ԭ�Ӱ뾶Խ������F�뾶��С��ͬ����Ԫ��ԭ�Ӵ����Ұ뾶��С������Na��Al�����뾶˳���ǣ�Na��Al��F���ʴ�Ϊ��Na��Al��F��
��2��C��N��Si����ۺ�����ֱ���H2CO3��HNO3��H2SiO3������˳����HNO3��H2CO3��H2SiO3���ʴ�Ϊ��HNO3��H2CO3��H2SiO3��
��3��H��O��Na��Cl�γɵļ��������Ӽ��ֺ��й��ۼ��Ļ�������NaOH�����ڼ��࣬NaClO��NaClO3��NaClO4���������࣬
�ʴ�Ϊ��NaOH��NaClO����NaClO3��NaClO4����
��4�����γ�18�����ӵķ�����SiH4��HCl��Ar��H2O2��F2��N2H4�ȣ��ʴ�Ϊ��SiH4��HCl��Ar��H2O2��F2��N2H4����д���ּ��ɣ���
��1�����ݵ��Ӳ�Խ�࣬ԭ�Ӱ뾶Խ������F�뾶��С��ͬ����Ԫ��ԭ�Ӵ����Ұ뾶��С������Na��Al�����뾶˳���ǣ�Na��Al��F���ʴ�Ϊ��Na��Al��F��
��2��C��N��Si����ۺ�����ֱ���H2CO3��HNO3��H2SiO3������˳����HNO3��H2CO3��H2SiO3���ʴ�Ϊ��HNO3��H2CO3��H2SiO3��
��3��H��O��Na��Cl�γɵļ��������Ӽ��ֺ��й��ۼ��Ļ�������NaOH�����ڼ��࣬NaClO��NaClO3��NaClO4���������࣬
�ʴ�Ϊ��NaOH��NaClO����NaClO3��NaClO4����
��4�����γ�18�����ӵķ�����SiH4��HCl��Ar��H2O2��F2��N2H4�ȣ��ʴ�Ϊ��SiH4��HCl��Ar��H2O2��F2��N2H4����д���ּ��ɣ���
���������⿼��Ԫ�ص��ƶϣ���Ŀ�ѶȲ�����Ԫ�������ڱ��е����ʿ��ƶϳ�Ԫ�ص����࣬���в����������ɵ�Ӧ�ã�ѧϰ��ע��������֪ʶ��

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
�����Ŀ








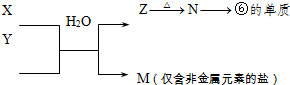
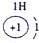
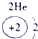
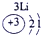
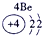
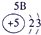
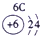
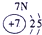
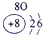
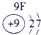
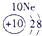
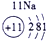





 ��ʾ����
��ʾ����