��Ŀ����
��14�֣��ƵĻ������ڹ�ũҵ���������ǵ��ճ����������Ź㷺��Ӧ�ã�����Na2O2������Ư����DZˮͧ�еĹ�������Na2O2��ǿ�����ԣ�H2���л�ԭ�ԣ�ijͬѧ����������ԭ��Ӧ��֪ʶ�Ʋ�Na2O2��H2�ܷ�Ӧ��Ϊ����֤���Ʋ�������ͬѧ��Ʋ���������ʵ�飬ʵ�鲽����������¡�
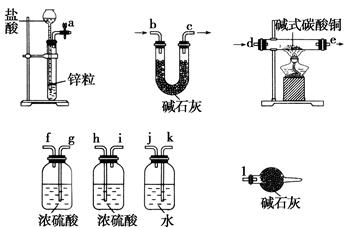
����1������ͼ��װ������ͼ�мг�����ʡ�ԣ������װ�õ������ԣ�Ȼ��װ��ҩƷ��
����2����K1��K2������������������װ��Na2O2��Ӳ�ʲ����ܵĹ����У�û�й۲쵽�κ�����
����3��������H2�Ĵ��Ⱥ�ȼ�ƾ��Ƽ��ȣ��۲쵽Ӳ�ʲ�������Na2O2���ۻ�������ɫ�ķ�ĩ��������˰�ɫ���壬�����������ͭδ����ɫ��
����4����Ӧ��ȥ�ƾ��ƣ���Ӳ�ʲ�������ȴ��ر�K1��
����������Ϣ�ش��������⣺
��1����װ��������Ҫ���װ�õ������ԡ��������K2֮ǰװ�������Եķ����������� ������Aװ���Ʊ�������ŵ�����������������д��1�㼴�ɣ���ʵ��������Aװ�û������Ʊ��������������� ��д��1�ּ��ɣ���
��2��ʢװCuSO4ҩƷ������������������ ��Bװ�õ������������������������� ��
��3����������������ȵ�ԭ�������������������������������������� ��
��4������װ��D��Ŀ������������������������ ��
��5��������ʵ����Ƴ�Na2O2��H2��Ӧ�Ļ�ѧ����ʽΪ������������������������ ��
��1���ر�K2����K1���ӳ���©���м�ˮ��������Һ������Թ�Һ�棬һ��ʱ���Һ���䣬
˵�����������ã��������ƻ��濪���ã�H2S�� CO2
��2������ܡ���ȥ�����е��Ȼ����ˮ���������
��3����ֹ������������ϼ��ȱ�ը�� ��
��4����������ˮ����
��5��Na2O2+H2 2NaOH
2NaOH
���������������1����װ���������K2֮ǰװ�������Եķ����ǹر�K2����K1���ӳ���©���м�ˮ��������Һ������Թ�Һ�棬һ��ʱ���Һ���䣬˵�����������ã�����Aװ���Ʊ�������ŵ����������ƻ��濪���ã�ʵ��������Aװ���Ʊ���������ϵ������ǿ�״������Һ�巴Ӧ����Ӧ������ȡ����ɵ�������ˮ�е��ܽ�Ȳ��������Ʊ���������H2S�� CO2����2�����������Ĺ���֪��ʢװCuSO4ҩƷ�����������Ǹ���ܣ�Bװ�õ������dz�ȥ�����е��Ȼ����ˮ��������ӡ���3����������������ȵ�ԭ���Ƿ�ֹ������������ϼ��ȱ�ը����4������װ��D��Ŀ���Ǽ�������ˮ���ɣ���5��������ʵ�����������֪�������Ʊ�Ϊ��ɫ���壬����ͭ������ɫ֤����ˮ���ɣ�֤�������������Ʒ�Ӧ�����������ƣ���ѧ����ʽΪNa2O2+H2 2NaOH��
2NaOH��
���㣺������������ʵ��ķ��������ۡ�


 ��
�� �⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ��
�⣬�����ܴ��� �������ӷ��ű�ʾ�����������ӵķ����� ��




 (x��y)Cu��xCO2��(x��2y��z)H2O
(x��y)Cu��xCO2��(x��2y��z)H2O