��Ŀ����
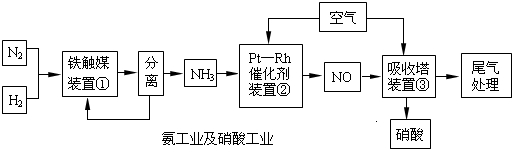
��ҵ�ϳɰ����Ʊ�����һ�����������,������ͼ17��ʾ��

��ش��������⣺
I.�ϳɰ�
(1)��֪��һ�����¶��½���װ�âٵĵ�����������(�����ۼ����Ȼ�ϣ����װ�âٳ����Ļ������ѹǿ֮��Ϊ5: 4��������ת����Ϊ________________________��
II.���ĽӴ�����ԭ��
��֪1 ����9000Cʱ��װ�â��з�Ӧ�У�

�������з�Ӧ�⣬����һ����������ã�
 ��
�� ,�����ܷ�������һ�������ķֽ⡣
,�����ܷ�������һ�������ķֽ⡣
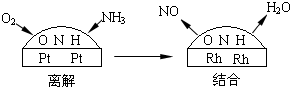
��֪2:��һ���Ͻ�����Ĵ�����Ϊ���ͽ�������̣���ͼ18��ʾ�����ڲ���NO��ˮ���ӵ���������С�������ڵ�����ԭ�ӽ��,ʹ��NO��ˮ�����ڲ������Ѹ�,���������С���ش��(2) (3)С�⣺

��2������Ȼ�ѧ����ʽ�� ��
�� =________________��
=________________��
��3����û��ʹ����һ��Ͻ����,�������������Ҫ����________��˵�������Է�Ӧ��________
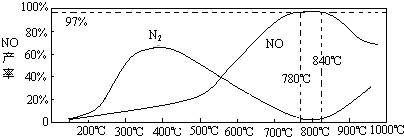
��4���¶ȶ�һ���������ʵ�Ӱ�죨ͼ19)

���¶ȴ���900¾ʱ��NO�IJ����½���ԭ��________________________(ѡ����ţ���
A.�ٽ���һ�������ķֽ� B.�ٽ��˰��ķֽ�
C.ʹ����һ�������ķ�Ӧƽ���ƶ������ɸ���N2
���𰸡�

����������

��ϰ��ϵ�д�
�����Ŀ
��ҵ�ϳɰ����Ʊ�����һ�������������������ͼ��

��1����ҵ����ʱ����ȡ������һ����ӦΪ��CO��g��+H2O��g��?CO2��g��+H2��g����t��ʱ����10L�ܱ������г���2mol CO��3molˮ��������Ӧ����ƽ�����ϵ��c��H2��=0.12mol?L-1������¶��´˷�Ӧ��ƽ�ⳣ��K= �������������
��2���ϳ����з�����ӦN2��g��+3H2��g��?2NH3��g����H��0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1 300�棨���������������=������
��3�������ڴ�����ȼ������һ�ֵ��ʺ�ˮ����ѧ�����ô�ԭ������Ƴɡ�����-������ȼ�ϵ�أ���ͨ�백���ĵ缫�� ����������������������������£��õ缫������Ӧ�ĵ缫��ӦʽΪ ��
��4���ð������������������ᣬ��β���е�NOx����Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ����������ﻹԭΪ������ˮ����Ӧ����Ϊ��
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ?mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ?mol-1
�����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ ��
��5��ij�о�С����ʵ�����ԡ�Ag-ZSM-5��Ϊ��������ý�NOת��ΪN2��ת�������¶ȱ仯�������ͼ����ͼ����������ʹ��CO���¶ȳ���775�棬����NO��ת���ʽ��ͣ�����ܵ�ԭ��Ϊ ����
=1�������£�Ӧ���Ƶ�����¶��� ���ң�


��1����ҵ����ʱ����ȡ������һ����ӦΪ��CO��g��+H2O��g��?CO2��g��+H2��g����t��ʱ����10L�ܱ������г���2mol CO��3molˮ��������Ӧ����ƽ�����ϵ��c��H2��=0.12mol?L-1������¶��´˷�Ӧ��ƽ�ⳣ��K=
��2���ϳ����з�����ӦN2��g��+3H2��g��?2NH3��g����H��0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1
| T/�� | T1 | 300 | T2 |
| K | 1.00��107 | 2.45��105 | 1.88��103 |
��4���ð������������������ᣬ��β���е�NOx����Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ����������ﻹԭΪ������ˮ����Ӧ����Ϊ��
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ?mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ?mol-1
�����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ
��5��ij�о�С����ʵ�����ԡ�Ag-ZSM-5��Ϊ��������ý�NOת��ΪN2��ת�������¶ȱ仯�������ͼ����ͼ����������ʹ��CO���¶ȳ���775�棬����NO��ת���ʽ��ͣ�����ܵ�ԭ��Ϊ
| n(NO) |
| n(CO) |



 ��3��NH3��O2�ڲ�ϵ���������´�145��Ϳ�ʼ��Ӧ��4NH3��g��+5O2��g��?4NO��g��+6H2O��g����H=-905kJ?mol-1����ͬ�¶���NO��������ͼ��ʾ���¶ȸ���900��ʱ��NO�����½���ԭ��
��3��NH3��O2�ڲ�ϵ���������´�145��Ϳ�ʼ��Ӧ��4NH3��g��+5O2��g��?4NO��g��+6H2O��g����H=-905kJ?mol-1����ͬ�¶���NO��������ͼ��ʾ���¶ȸ���900��ʱ��NO�����½���ԭ��
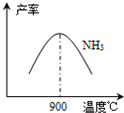 ��3��N2��H2���������������´�145��Ϳ�ʼ��Ӧ����ͬ�¶���NH3������ͼ��ʾ���¶ȸ���900��ʱ��NH3�����½���ԭ��
��3��N2��H2���������������´�145��Ϳ�ʼ��Ӧ����ͬ�¶���NH3������ͼ��ʾ���¶ȸ���900��ʱ��NH3�����½���ԭ��