��Ŀ����
X��Y���Ƿ����廯�����Ϊ����ʳ���㾫���㷺���ڻ�ױƷ���ǹ�����ζƷ�У�1 mol��Xˮ��õ�1 mol��Y��1 mol��CH2CH2OH��X��Y����Է���������������200����ȫȼ�ն�ֻ����CO2��H2O����x������̼����Ԫ���ܵ������ٷֺ���ԼΪ81.8����
(1)1��Y������Ӧ����________����ԭ�ӣ�
(2)X�ķ���ʽ��________��
(3)G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ��÷�����A�ϳ�G·�����£�
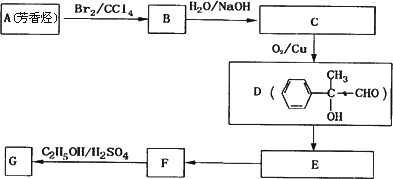
��д��A�Ľṹ��ʽ________��
��E��F�ķ�Ӧ������________��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ________��
��д�����з�������������F��ͬ���칹��Ľṹ��ʽ��________��
���������ڳ��˱��������������ṹ���ұ�������2����λȡ������
����һ�������£������ʼ�����������Һ����������ӦҲ�ܺ�FeCl3��Һ������ɫ��Ӧ��
������
|
����(1)2(1��) ����(2)C11H12O2(2��) ����(3)�� ��������ȥ��(1��) ���� ������ |

��10�֣�X��Y���Ƿ����廯�����Ϊ����ʳ���㾫���㷺���ڻ�ױƷ���ǹ�����ζƷ�С�1mol Xˮ��õ�1 molY��1mol CH3CH2OH��X��Y����Է���������������200����ȫȼ�ն�ֻ����CO2��H2O����x������̼����Ԫ���ܵ������ٷֺ���ԼΪ81��8����
��1��1��Y������Ӧ���� ����ԭ�ӡ�
��2��X�ķ���ʽ�� ��
��3��G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ��÷�����A�ϳ�G·�����£�
 |
��д��A�Ľṹ��ʽ ��
��E��F�ķ�Ӧ������ ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��д�����з�������������F��ͬ���칹��Ľṹ��ʽ�� ��
i�������ڳ��˱��������������ṹ���ұ�������2����λȡ������
ii��һ�������£������ʼ�����������Һ����������ӦҲ�ܺ�FeCl3��Һ������ɫ��Ӧ��



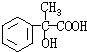
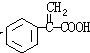 +H2O
+H2O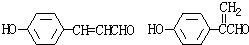
 ��
��
 F�ķ�Ӧ������ ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
F�ķ�Ӧ������ ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��