��Ŀ����
��1��0.25molij����A��������15g��A����Է�������Ϊ______��
��2����ͬ��ͬѹͬ����������£�H2������A������֮����1��8����A��Ħ������Ϊ______��
��3����25�桢101kPa�������£�ͬ������CH4��A��������֮����15��8����A��Ħ������Ϊ______��
��4������ͬ�ݻ����ܷ�����A��B����25���£�A�г���a g A���壬B�г���a g CH4���壬A��B�ڵ�ѹǿ֮����4��11����A��Ħ������Ϊ______��
��5����ͬ��ͬѹ�£���ͬ�����CH4������A���ܶ�֮��Ϊ1��4����A��Ħ������Ϊ______��
��6���ڱ�״���£�����A���ܶ���3.170g/L����A��Ħ������Ϊ______��
�⣺��1��M= ����Ħ����������ֵ�ϵ�������Է���������������Է�������Ϊ60���ʴ�Ϊ60��
����Ħ����������ֵ�ϵ�������Է���������������Է�������Ϊ60���ʴ�Ϊ60��
��2����ͬ��ͬѹͬ����������£� =
= ������M 2=16 g/mol
������M 2=16 g/mol
�ʴ�Ϊ16 g/mol
��3����25�桢101kPa�������£� =
= ������M 2=30 g/mol
������M 2=30 g/mol
�ʴ�Ϊ��30 g/mol
��4������ͬ�¶ȣ���ͬ�ݻ��������ڣ������ѹǿ֮�ȵ��������ʵ���֮�ȣ� =
= ������M1=44 g/mol
������M1=44 g/mol
�ʴ�Ϊ44 g/mol
��5����ͬ��ͬѹ�£�����Ħ�������ͬ��
 =
= ��
��
����M2=64 g/mol
�ʴ�Ϊ64 g/mol
��6���ڱ�״���£�
 =3.170g/L ����M=71 g/mol���ʴ�Ϊ71 g/mol��
=3.170g/L ����M=71 g/mol���ʴ�Ϊ71 g/mol��
��������1�������A��Ħ���������ٸ���Ħ����������Է��������Ĺ�ϵ������
��2��������ͬ��ͬѹͬ����������£������������Ħ�������Ĺ�ϵ�жϣ�
��3��������ͬ�����£�������Ħ������������Ĺ�ϵ���㣻
��4���ȸ�����ͬ�¶ȡ���������壬��ѹǿ֮�������ʵ���֮�ȵĹ�ϵ��Ȼ��ȷ����ͬ����ʱ���ܶ�֮����Ħ������֮�ȵĹ�ϵ���㣻
��5������ͬ��ͬѹ�����£��ܶ�֮����Ħ������֮�ȵĹ�ϵ���㣻
��6���ڱ�״���£������ܶ���Ħ������������Ħ������Ĺ�ϵ���㣻
���������⿼���˲�ͬ�����£�������Ħ��������������ܶȵ���������Ĺ�ϵ�����ؿ���ѧ�������ʵ����ĸ�������Ӻ��ں������⣮
 ����Ħ����������ֵ�ϵ�������Է���������������Է�������Ϊ60���ʴ�Ϊ60��
����Ħ����������ֵ�ϵ�������Է���������������Է�������Ϊ60���ʴ�Ϊ60����2����ͬ��ͬѹͬ����������£�
 =
= ������M 2=16 g/mol
������M 2=16 g/mol �ʴ�Ϊ16 g/mol
��3����25�桢101kPa�������£�
 =
= ������M 2=30 g/mol
������M 2=30 g/mol �ʴ�Ϊ��30 g/mol
��4������ͬ�¶ȣ���ͬ�ݻ��������ڣ������ѹǿ֮�ȵ��������ʵ���֮�ȣ�
 =
= ������M1=44 g/mol
������M1=44 g/mol �ʴ�Ϊ44 g/mol
��5����ͬ��ͬѹ�£�����Ħ�������ͬ��

 =
= ��
������M2=64 g/mol
�ʴ�Ϊ64 g/mol
��6���ڱ�״���£�
 =3.170g/L ����M=71 g/mol���ʴ�Ϊ71 g/mol��
=3.170g/L ����M=71 g/mol���ʴ�Ϊ71 g/mol����������1�������A��Ħ���������ٸ���Ħ����������Է��������Ĺ�ϵ������
��2��������ͬ��ͬѹͬ����������£������������Ħ�������Ĺ�ϵ�жϣ�
��3��������ͬ�����£�������Ħ������������Ĺ�ϵ���㣻
��4���ȸ�����ͬ�¶ȡ���������壬��ѹǿ֮�������ʵ���֮�ȵĹ�ϵ��Ȼ��ȷ����ͬ����ʱ���ܶ�֮����Ħ������֮�ȵĹ�ϵ���㣻
��5������ͬ��ͬѹ�����£��ܶ�֮����Ħ������֮�ȵĹ�ϵ���㣻
��6���ڱ�״���£������ܶ���Ħ������������Ħ������Ĺ�ϵ���㣻
���������⿼���˲�ͬ�����£�������Ħ��������������ܶȵ���������Ĺ�ϵ�����ؿ���ѧ�������ʵ����ĸ�������Ӻ��ں������⣮

��ϰ��ϵ�д�
�����Ŀ
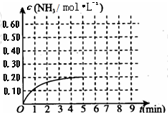 ��2010?��̨һģ��ij�¶�ʱ����һ�ݻ�Ϊ1L���ܱ������У�����0.4mol��N2��1.2mol��H2����һ�������·������·�Ӧ��N2��g��+3H2��g��?2NH3��g����H��0��5minʱ�ﵽƽ�⣬��Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺��1��������ͼ������ӷ�Ӧ��ʼ��ƽ��ʱ��ƽ����Ӧ����v��N2��=
��2010?��̨һģ��ij�¶�ʱ����һ�ݻ�Ϊ1L���ܱ������У�����0.4mol��N2��1.2mol��H2����һ�������·������·�Ӧ��N2��g��+3H2��g��?2NH3��g����H��0��5minʱ�ﵽƽ�⣬��Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺��1��������ͼ������ӷ�Ӧ��ʼ��ƽ��ʱ��ƽ����Ӧ����v��N2��=