ЬтФПФкШн
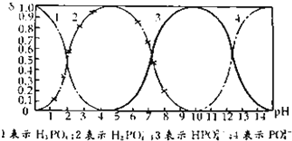 СзЫсЃЈH3PO4ЃЉдкШмвКжаФмЙЛвдH3PO4ЁЂH2PO4-ЁЂHPO42-КЭPO43-ЫФжжСЃзгЕФаЮЪНДцдкЃЌЕБШмвКжаЕФpHЗЂЩњБфЛЏЪБЃЌЦфжаШЮвЛжжСЃзгЕФЮяжЪЕФСПеМЫФжжСЃзгзмЮяжЪЕФСПЕФЗжЪ§ІФвВПЩФмЗЂЩњБфЛЏЃЎШчЭМЫљЪОЪЧH3PO4ШмвКжаЃЌИїжжСЃзгЕФЮяжЪЕФСПЗжЪ§ІФЫцpHБфЛЏЧњЯпЃК
СзЫсЃЈH3PO4ЃЉдкШмвКжаФмЙЛвдH3PO4ЁЂH2PO4-ЁЂHPO42-КЭPO43-ЫФжжСЃзгЕФаЮЪНДцдкЃЌЕБШмвКжаЕФpHЗЂЩњБфЛЏЪБЃЌЦфжаШЮвЛжжСЃзгЕФЮяжЪЕФСПеМЫФжжСЃзгзмЮяжЪЕФСПЕФЗжЪ§ІФвВПЩФмЗЂЩњБфЛЏЃЎШчЭМЫљЪОЪЧH3PO4ШмвКжаЃЌИїжжСЃзгЕФЮяжЪЕФСПЗжЪ§ІФЫцpHБфЛЏЧњЯпЃКЃЈ1ЃЉЩшСзЫсзмХЈЖШЮЊcЃЈзмЃЉЃЌаДГіcЃЈзмЃЉгыИїСЃзгХЈЖШМфЕФЙиЯЕЪН
ЃЈ2ЃЉЯђNa3PO4ШмвКжаж№ЕЮЕЮШыЯЁбЮЫсЃЌдкЕЮМгЙ§ГЬжаЃЌЪМжеДцдкЕШЪНЃКcЃЈNa+ЃЉ+cЃЈH+ЃЉ=
ЃЈ3ЃЉгЩЭМПЩжЊЃЌNa2HPO4ШмвКГЪМюадЃЌЦфдвђЪЧ
ЃЈ4ЃЉЯђNa3PO4ЯЁШмвКжаМгШыNa3PO4ЙЬЬхЃЌ
| c(Na+) |
| c(PO43-) |
ПМЕуЃКРызгХЈЖШДѓаЁЕФБШНЯ,ШѕЕчНтжЪдкЫЎШмвКжаЕФЕчРыЦНКт
зЈЬтЃКЕчРыЦНКтгыШмвКЕФpHзЈЬт
ЗжЮіЃКЃЈ1ЃЉДгЮяСЯЪиКуЕФНЧЖШЗжЮіЃЛ
ЃЈ2ЃЉДгЕчКЩЪиКуЕФНЧЖШЗжЮіЃЛШмвКжаH2PO4-діМгЃЌдкpHДг8НЕЕН6ЕФЙ§ГЬжаHPO42-МѕЩйЃЌЫљвдЪЧШмвКжаЕФHPO42-КЭЧтРызгЗЂЩњЗДгІЃЛ
ЃЈ3ЃЉИљОнЭМЯѓЗжЮіЃЌЕБШмвКжаДцдкHPO4-ЪБШмвКЕФpHжЕШЗЖЈШмвКЕФЫсМюадЃЌДгЖјШЗЖЈHPO4-ЕФЕчРыГЬЖШКЭЫЎНтГЬЖШЕФЯрЖдДѓаЁЃЛ
ЃЈ4ЃЉЯШИљОнСзЫсФЦбЮЕФРраЭШЗЖЈФЦРызгКЭСзЫсИљРызгЕФЙиЯЕЃЌдйЬМЫсИљРызгХЈЖШЕФБфЛЏРДЗжЮіЃЎ
ЃЈ2ЃЉДгЕчКЩЪиКуЕФНЧЖШЗжЮіЃЛШмвКжаH2PO4-діМгЃЌдкpHДг8НЕЕН6ЕФЙ§ГЬжаHPO42-МѕЩйЃЌЫљвдЪЧШмвКжаЕФHPO42-КЭЧтРызгЗЂЩњЗДгІЃЛ
ЃЈ3ЃЉИљОнЭМЯѓЗжЮіЃЌЕБШмвКжаДцдкHPO4-ЪБШмвКЕФpHжЕШЗЖЈШмвКЕФЫсМюадЃЌДгЖјШЗЖЈHPO4-ЕФЕчРыГЬЖШКЭЫЎНтГЬЖШЕФЯрЖдДѓаЁЃЛ
ЃЈ4ЃЉЯШИљОнСзЫсФЦбЮЕФРраЭШЗЖЈФЦРызгКЭСзЫсИљРызгЕФЙиЯЕЃЌдйЬМЫсИљРызгХЈЖШЕФБфЛЏРДЗжЮіЃЎ
НтД№ЃК
НтЃКЃЈ1ЃЉСзЫсЃЈH3PO4ЃЉдкШмвКжаФмЙЛвдH3PO4ЁЂH2PO4-ЁЂHPO42-ЁЂКЭPO43-ЫФжжСЃзгаЮЪНДцдкЃЌЮоТлвдКЮжжРызгДцдкЃЌЕЋзмЕФPдзгзмЪ§гыдСзЫсжаPдзгЯрЕШЃЌЫљвдcЃЈзмЃЉ=cЃЈH3PO4ЃЉ+cЃЈH2PO4-ЃЉ+cЃЈHPO42-ЃЉ+cЃЈPO43-ЃЉЃЌ
ЙЪД№АИЮЊЃКcЃЈзмЃЉ=cЃЈH3PO4ЃЉ+cЃЈH2PO4-ЃЉ+cЃЈHPO42-ЃЉ+cЃЈPO43-ЃЉЃЛ
ЃЈ2ЃЉШмвКДцдкЕчКЩЪиКуЃЌЮЊcЃЈNa+ЃЉ+cЃЈH+ЃЉ=cЃЈOH-ЃЉ+cЃЈH2PO4-ЃЉ+2cЃЈHPO42-ЃЉ+3cЃЈPO43-ЃЉ+cЃЈCl-ЃЉЃЌИљОнЭМЯѓжЊЃЌЕБЯђШмвКжаЕЮМгЯЁбЮЫсЪБЃЌШмвКжаH2PO4-діМгЃЌHPO42-МѕЩйЃЌЫљвдЪЧШмвКжаЕФHPO42-КЭЧтРызгЗЂЩњЩњГЩH2PO4-ЃЌРызгЗНГЬЪНЮЊЃКHPO42-+H+ЈTH2PO4-ЃЌ
ЙЪД№АИЮЊЃКcЃЈOH-ЃЉ+cЃЈH2PO4-ЃЉ+2cЃЈHPO42-ЃЉ+3cЃЈPO43-ЃЉ+cЃЈCl-ЃЉЃЛHPO42-+H+=H2PO4-ЃЛ
ЃЈ3ЃЉNa2HPO4ШмвКГЪМюадЃЌИљОнЭМЯѓжЊЃЌЕБШмвКжаДцдкHPO4-ЪБЃЌШмвКГЪМюадЃЌЫЕУїHPO4-дкШмвКжаЕФЫЎНтГЬЖШДѓгкЕчРыГЬЖШЃЌЕМжТШмвКжаЧтРызгХЈЖШаЁгкЧтбѕИљРызгЕФХЈЖШЃЌЙЪД№АИЮЊЃКHPO42-ЕФЫЎНтДѓгкЦфЕчРыЃЛ
ЃЈ4ЃЉСзЫсФЦЪЧЧПМюШѕЫсбЮЃЌЫљвдШѕЫсИљРызгФмЗЂЩњЫЎНтЃЌЕМжТШмвКжа
ЃО3ЃЛ PO43-+H2O?HPO42-+OH-ЃЌЕБЯђШмвКжаМгШыNa3PO4ЙЬЬхЃЌЕМжТШмвКжаСзЫсИљРызгЕФХЈЖШдіДѓЃЌЙЪ
ЕФжЕМѕаЁЃЌ
ЙЪД№АИЮЊЃКМѕаЁЃЎ
ЙЪД№АИЮЊЃКcЃЈзмЃЉ=cЃЈH3PO4ЃЉ+cЃЈH2PO4-ЃЉ+cЃЈHPO42-ЃЉ+cЃЈPO43-ЃЉЃЛ
ЃЈ2ЃЉШмвКДцдкЕчКЩЪиКуЃЌЮЊcЃЈNa+ЃЉ+cЃЈH+ЃЉ=cЃЈOH-ЃЉ+cЃЈH2PO4-ЃЉ+2cЃЈHPO42-ЃЉ+3cЃЈPO43-ЃЉ+cЃЈCl-ЃЉЃЌИљОнЭМЯѓжЊЃЌЕБЯђШмвКжаЕЮМгЯЁбЮЫсЪБЃЌШмвКжаH2PO4-діМгЃЌHPO42-МѕЩйЃЌЫљвдЪЧШмвКжаЕФHPO42-КЭЧтРызгЗЂЩњЩњГЩH2PO4-ЃЌРызгЗНГЬЪНЮЊЃКHPO42-+H+ЈTH2PO4-ЃЌ
ЙЪД№АИЮЊЃКcЃЈOH-ЃЉ+cЃЈH2PO4-ЃЉ+2cЃЈHPO42-ЃЉ+3cЃЈPO43-ЃЉ+cЃЈCl-ЃЉЃЛHPO42-+H+=H2PO4-ЃЛ
ЃЈ3ЃЉNa2HPO4ШмвКГЪМюадЃЌИљОнЭМЯѓжЊЃЌЕБШмвКжаДцдкHPO4-ЪБЃЌШмвКГЪМюадЃЌЫЕУїHPO4-дкШмвКжаЕФЫЎНтГЬЖШДѓгкЕчРыГЬЖШЃЌЕМжТШмвКжаЧтРызгХЈЖШаЁгкЧтбѕИљРызгЕФХЈЖШЃЌЙЪД№АИЮЊЃКHPO42-ЕФЫЎНтДѓгкЦфЕчРыЃЛ
ЃЈ4ЃЉСзЫсФЦЪЧЧПМюШѕЫсбЮЃЌЫљвдШѕЫсИљРызгФмЗЂЩњЫЎНтЃЌЕМжТШмвКжа
| c(Na+) |
| c(PO43-) |
| c(Na+) |
| c(PO43-) |
ЙЪД№АИЮЊЃКМѕаЁЃЎ
ЕуЦРЃКБОЬтПМВщСЫШмвКжадзгЪиКуЁЂбЮРрЕФЫЎНтЖдФбШмЕчНтжЪЕФШмНтЦНКтКЭЫсМюЗДгІЕШжЊЪЖЕуЃЌЖдбЇЩњЪщаДЛЏбЇЗДгІЗНГЬаЮЪНЕФФмСІЁЂЖСЭМНтЮіКЭЗНАИЦРМлФмСІЃЌвдМАЛЏбЇЦНКтжЊЪЖЕФзлКЯгІгУФмСІвЊЧѓНЯИпЃЌЕЋФбЖШВЛЪЧКмДѓЃЎ

СЗЯАВсЯЕСаД№АИ
ЯрЙиЬтФП
ЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
| AЁЂГЃЮТЯТЃЌ20L pH=12ЕФNa2CO3ШмвКжаКЌгаЕФOH-РызгЪ§ЮЊ0.2NA | ||
| BЁЂжаКЭЕШЬхЛ§ЁЂЕШЮяжЪЕФСПХЈЖШбЮЫсКЭДзЫсШмвКЃЌбЮЫсЫљашNaOHШмвКЖргкДзЫс | ||
CЁЂЯђ0.1mol/L NH3?H2OШмвКжаМгШыЩйСПNH4ClЙЬ ЬхЃЌШмвКжа
| ||
| DЁЂвЛЖЈЮТЖШЯТЃЌ10mL 0.50mol?L-1 NH4ClШмвКгы20mL 0.25mol?L-1 NH4ClШмвККЌNH4+ЮяжЪЕФСПЯрЭЌ |
 НЋMgКЭAlЗлФЉОљдШЛьКЯЃЌМгШыЕН100mLФГХЈЖШЕФСђЫсжаЃЌВњЩњЧтЦјЕФЬхЛ§ЃЈБъзМзДПіЯТЃЉгыМгШыЗлФЉЕФжЪСПЙиЯЕШчЭМЫљЪОЃК
НЋMgКЭAlЗлФЉОљдШЛьКЯЃЌМгШыЕН100mLФГХЈЖШЕФСђЫсжаЃЌВњЩњЧтЦјЕФЬхЛ§ЃЈБъзМзДПіЯТЃЉгыМгШыЗлФЉЕФжЪСПЙиЯЕШчЭМЫљЪОЃК вбжЊФГДМШМСЯКЌгаЬМЁЂЧтЁЂбѕШ§жждЊЫиЃЎЮЊСЫВтЖЈетжжШМСЯжаЬМКЭЧтСНжждЊЫиЕФжЪСПБШЃЌПЩНЋЦјЬЌШМСЯЗХШызуСПЕФбѕЦјжаШМЩеЃЌВЂЪЙВњЩњЕФЦјЬхШЋВПЭЈШыШчЭМЫљЪОЕФзАжУЃЌЕУЕНШчЯТБэЫљСаЕФЪЕбщНсЙћЃЈМйЩшВњЩњЕФЦјЬхЭъШЋБЛЮќЪеЃЉЃК
вбжЊФГДМШМСЯКЌгаЬМЁЂЧтЁЂбѕШ§жждЊЫиЃЎЮЊСЫВтЖЈетжжШМСЯжаЬМКЭЧтСНжждЊЫиЕФжЪСПБШЃЌПЩНЋЦјЬЌШМСЯЗХШызуСПЕФбѕЦјжаШМЩеЃЌВЂЪЙВњЩњЕФЦјЬхШЋВПЭЈШыШчЭМЫљЪОЕФзАжУЃЌЕУЕНШчЯТБэЫљСаЕФЪЕбщНсЙћЃЈМйЩшВњЩњЕФЦјЬхЭъШЋБЛЮќЪеЃЉЃК вбжЊвЛЖЈСПЕФгаЛњЮяAдкбѕЦјжаЭъШЋШМЩеЃЌжЛЩњГЩ0.2mol CO2КЭ0.3mol H2OЃЌЪдЛиД№ЯТСаЮЪЬтЃК
вбжЊвЛЖЈСПЕФгаЛњЮяAдкбѕЦјжаЭъШЋШМЩеЃЌжЛЩњГЩ0.2mol CO2КЭ0.3mol H2OЃЌЪдЛиД№ЯТСаЮЪЬтЃК