��Ŀ����
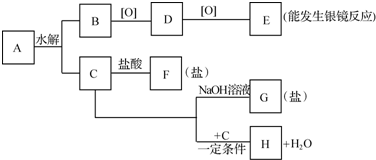
17��A��B��C��D��E��FΪ6�ֶ�����Ԫ�أ���ԭ��������������B��C��Dͬ���ڣ�D��Eͬ���壬A��B������ԭ�Ӹ�����4��1�γɻ�����ף��Ҽ����к���10�����ӣ�A��C�γɻ������ң��ҿ���E������������ˮ���ﰴ�����ʵ���֮��2��1��Ӧ�γ��α�����1��BԪ����Ԫ�����ڱ��е�λ���ǵڶ����ڵڢ�A�壻
��2���ҵĵ���ʽ
 ��
����3��A��D�γɵļȺ����Լ��ֺ��зǼ��Լ��Ĺ��ۻ�����Ľṹʽ��H-O-O-H��
��4������������Һ�м������ŨBa��OH��2��Һ�����ȣ���Ӧ�����ӷ���ʽ��2NH4++SO42-+Ba2++2OH-$\frac{\underline{\;\;��\;\;}}{\;}$2NH3��+BaSO4��+2H2O��
��5���Һ�Ԫ��D�ĵ��ʷ�Ӧ��ij���������Ļ�����д���˷�Ӧ�Ļ�ѧ����ʽ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
���� A��B��C��D��E��FΪ6�ֶ�����Ԫ�أ���ԭ��������������A��B������ԭ�Ӹ�����4��1�γɻ�����ף��Ҽ����к���10�����ӣ����ΪCH4��AΪHԪ�ء�BΪCԪ�أ�
A��C�γɻ������ң��ҿ���E������������ˮ���ﰴ�����ʵ���֮��2��1��Ӧ�γ��α�������ΪNH3����Ϊ��Σ�E������������ˮ����Ϊ��Ԫ�ᣬ��ӦΪ��NH4��2SO4����CΪNԪ�أ�EΪSԪ�أ�D��Eͬ���壬DΪOԪ�أ�
FΪ������Ԫ�أ���ԭ����������S��16����EΪClԪ�أ��ݴ˽��н��
��� �⣺A��B��C��D��E��FΪ6�ֶ�����Ԫ�أ���ԭ��������������A��B������ԭ�Ӹ�����4��1�γɻ�����ף��Ҽ����к���10�����ӣ����ΪCH4��AΪHԪ�ء�BΪCԪ�أ�A��C�γɻ������ң��ҿ���E������������ˮ���ﰴ�����ʵ���֮��2��1��Ӧ�γ��α�������ΪNH3����Ϊ��Σ�E������������ˮ����Ϊ��Ԫ�ᣬ��ӦΪ��NH4��2SO4����CΪNԪ�أ�EΪSԪ�أ�D��Eͬ���壬DΪOԪ�أ�FΪ������Ԫ�أ���ԭ����������S��16����EΪClԪ�أ�
��1��BΪCԪ�أ���ԭ������Ϊ6������Ԫ�����ڱ��еڶ����ڵڢ�A�壬
�ʴ�Ϊ���ڶ����ڵڢ�A�壻
��2����Ϊ����������Ϊ���ۻ���������ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��3��O��H�γɵļȺ����Լ��ֺ��зǼ��Լ��Ĺ��ۻ�����Ľṹʽ��ѧʽΪH2O2����ṹʽΪ��H-O-O-H��
�ʴ�Ϊ��H-O-O-H��
��4����Ϊ����泥��������������Һ�м������ŨBa��OH��2��Һ�����ȣ���Ӧ���ɰ��������ᱵ������ˮ���÷�Ӧ�����ӷ���ʽΪ��2NH4++SO42-+Ba2++2OH-$\frac{\underline{\;\;��\;\;}}{\;}$2NH3��+BaSO4��+2H2O��
�ʴ�Ϊ��2NH4++SO42-+Ba2++2OH-$\frac{\underline{\;\;��\;\;}}{\;}$2NH3��+BaSO4��+2H2O��
��5����Ϊ������Ԫ��D�ĵ���Ϊ������������������Ӧ����һ��������ˮ���÷�Ӧ��ij���������Ļ�������Ӧ�Ļ�ѧ����ʽΪ��4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6 H2O��
�ʴ�Ϊ��4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6 H2O��
���� ���⿼����λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���������ԭ�ӽṹ��Ԫ�������ɡ�Ԫ�����ڱ��Ĺ�ϵΪ���ؼ���������ؿ���ѧ���ķ���������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��������258 | B�� | ��������101 | C�� | ��������157 | D�� | ��������359 |
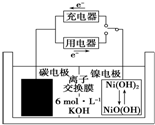 һ��̼�����ܹ������������������ε�أ���ͼ��ʾ����̼�缫���õ�صĵ������ҺΪ6mol•L-1��KOH��Һ������˵����ȷ���ǣ�������
һ��̼�����ܹ������������������ε�أ���ͼ��ʾ����̼�缫���õ�صĵ������ҺΪ6mol•L-1��KOH��Һ������˵����ȷ���ǣ�������| A�� | ���ʱ��������������Ӧ | |
| B�� | ���ʱ��̼�缫���Դ���������� | |
| C�� | �ŵ�ʱ̼�缫��ӦΪH2-2e-�T2H+ | |
| D�� | �ŵ�ʱ���缫��ӦΪNiO��OH��+H2O+e-�TNi��OH��2+OH- |
�ٹ�����п��18mol/L������Һ��Ӧ
�ڹ����������������ĵ����ڴ������ں�һ�������³�ַ�Ӧ
�ۼ���������Ũ���������MnO2 ��Ӧ
�ܼ��������¹���ͭ��Ũ���ᷴӦ
�ݹ���ϡ�������״ʯ��ʯ��Ӧ��
| A�� | �ڢۢܢ� | B�� | �ڢۢ� | C�� | �٢ۢ� | D�� | �٢ڢۢܢ� |
| A�� | $\frac{3a}{2B}$mol/L | B�� | $\frac{a}{27B}$mol/L | C�� | $\frac{a}{18B}$mol/L | D�� | $\frac{2a}{81B}$mol/L |
| ������ | Ag+ Na+ |
| ������ | NO3- SO42- Cl- |

�ݴ˻ش��������⣺
��1��MΪ��Դ�ĸ�������д���������������ס��ҵ���ʷֱ�ΪNaCl��AgNO3����д��ѧʽ����
��2������缫f�����ɵ������ڱ�״���µ������1.4L��
��3��д�����ձ��ĵ����ܷ�Ӧ����ʽ��4AgNO3+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$4Ag+O2��+4HNO3��
��4��Ҫʹ���ָ���ԭ����״̬��Ӧ����2.25�ˣ�����������H2O������д��ѧʽ��