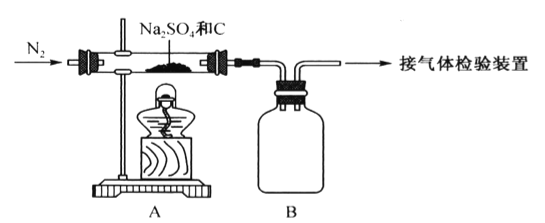
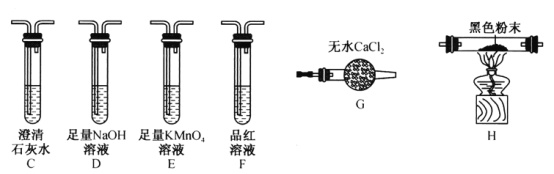
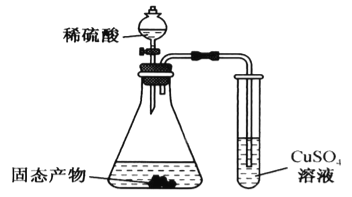
��Ŀ����
����Ŀ������73g�Ȼ��⣨HCl�����壬��
(1)�������Ħ������Ϊ_____________��
(2)����������ʵ���Ϊ________mol��������������Ϊ_________����
(3)�������ڱ�״���µ����Ϊ_____________L��
(4)�����ڷ�����ᴿ���ʵķ����Тٹ��� �ڽᾧ �������ܷ�Һ������ţ���
a.��ȥʯ��ˮ��������̼��ƿ���_________��
b.��ȥ�Ȼ����л��еĵⵥ�ʣ������ɹ�̬��Ϊ��̬��__________��
c.����ˮ�뱽�Ļ����____________��
(5)�ڱ�״���£����1.92gij��������Ϊ672ml������������Է�������__________��
��6��N2��CO2��SO2���������������Ϊ7��11��16ʱ�����ǵķ��Ӹ�����Ϊ_______�����ʵ���֮��Ϊ___________��ͬ��ͬѹ�������Ϊ___________��
���𰸡�36.5g/mol22NA44.8�٢ۢ�641��1:11��1:11��1:1
��������
(1)HCl�������Է���������36.5����������Ħ������Ϊ36.5g/mol��
(2)����n��m/M��֪����������ʵ���Ϊ73g��36.5g/mol��2mol��������������Ϊ2NA����
(3)��״���������Ħ�������22.4L/mol�����Ը������ڱ�״���µ����Ϊ2mol��22.4L/mol��44.8L��
(4)a.̼��Ʋ�����ˮ�����ȥʯ��ˮ��������̼��ƿ�����Ҫ���ˣ���ѡ�٣�
b.�����ɹ�̬��Ϊ��̬�����ȥ�Ȼ����л��еĵⵥ�ʿ���������������ѡ�ۣ�
c.��������ˮ������ˮ�뱽�Ļ������Ҫ��Һ������ѡ�ܡ�
(5)�ڱ�״���£����1.92gij��������Ϊ672mL�����ʵ�����0.672L��22.4L/mol��0.03mol�����Դ��������Է�������Ϊ1.92��0.03��64��
(6)N2��CO2��SO2���������������Ϊ7��11��16ʱ�����ǵķ��Ӹ�����Ϊ![]() �������ʵ���֮��Ϊ1��1��1��ͬ��ͬѹ�������Ϊ1��1��1��
�������ʵ���֮��Ϊ1��1��1��ͬ��ͬѹ�������Ϊ1��1��1��

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�