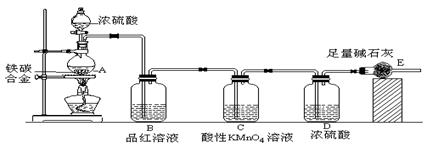
��Ŀ����
(10��)�ɶ�������������С������˹��ڡ����ý������ᷴӦ����ʵ�飬�ó���һЩ��Ȥ���뷨������������ǣ����һЩ�жϡ���5.4g��ƬͶ��500mL 0.5 mol��L-1��������Һ�У���ͼΪ��Ӧ���������뷴Ӧʱ��Ĺ�ϵͼ��
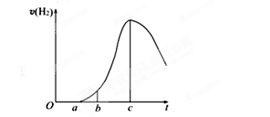
��1������ͼ�����������ȷ����_��_____(2��)
��a��c�β����������������ӽϿ�����DZ��������Ĥ���ܽ⣬�ӿ��˷�Ӧ������
��a��c�β����������������ӽϿ�����Ǹ÷�Ӧ���ȣ������¶����ߣ��ӿ��˷�Ӧ
��c�Ժ����������������½���������Ƭ����������ȫ
��2����дO��a������Ӧ�����ӷ���ʽ____________________________________(2��)
��3���������������䣬�ֻ���500mL1mol��L-1���ᣬ�������������ձ��ʹ��500mL 0.5mol��L-1����Ŀ죬���ܵ�ԭ����_�ڢ�_______(2��)
��������c(H+)��Ũ�ȸ��� ��Cl���д����� ��SO42���д�����
��SO42�����軯���� ��Al3+�д�����
��4��������ѧ����Ҫ�ӿ���Ƭ��������Һ��H2�ķ�Ӧ���ʣ�С���Ա���һϵ�з��������磺�ټ���ij�ִ������ڵμ�����CuSO4��Һ���۽���Ƭ����100���������������Ũ����18mol/L���ݼ�������ˮ����������KNO3��Һ����_____________��(2��)
���У�����Ϊ�������ķ�����_____________(2��)���������ٲ���һ�ֺ�������������հעߴ���

��1������ͼ�����������ȷ����_��_____(2��)
��a��c�β����������������ӽϿ�����DZ��������Ĥ���ܽ⣬�ӿ��˷�Ӧ������
��a��c�β����������������ӽϿ�����Ǹ÷�Ӧ���ȣ������¶����ߣ��ӿ��˷�Ӧ
��c�Ժ����������������½���������Ƭ����������ȫ
��2����дO��a������Ӧ�����ӷ���ʽ____________________________________(2��)
��3���������������䣬�ֻ���500mL1mol��L-1���ᣬ�������������ձ��ʹ��500mL 0.5mol��L-1����Ŀ죬���ܵ�ԭ����_�ڢ�_______(2��)
��������c(H+)��Ũ�ȸ��� ��Cl���д����� ��SO42���д�����
��SO42�����軯���� ��Al3+�д�����
��4��������ѧ����Ҫ�ӿ���Ƭ��������Һ��H2�ķ�Ӧ���ʣ�С���Ա���һϵ�з��������磺�ټ���ij�ִ������ڵμ�����CuSO4��Һ���۽���Ƭ����100���������������Ũ����18mol/L���ݼ�������ˮ����������KNO3��Һ����_____________��(2��)
���У�����Ϊ�������ķ�����_____________(2��)���������ٲ���һ�ֺ�������������հעߴ���
��1���ۣ�2�֣� ��2��Al2O3+6H+="2" Al3++3H2O��2�֣�
��3���ڢܣ�2�֣� ��4���������¶ȣ�2�֣� �ܢݢޣ�2�֣�
��3���ڢܣ�2�֣� ��4���������¶ȣ�2�֣� �ܢݢޣ�2�֣�
��1���ڷ�Ӧ�ĺ��ڣ����������Ũ�����ͣ����·�Ӧ�������͡����ǹ��壬���������ٲ���Ӱ�췴Ӧ���ʣ����Ԣ��Ǵ���ģ��������ȷ�ģ���ѡ��.
(2)���ǻ��õĽ������ױ���������һ�����ܵ�����Ĥ�����Կ�ʼû��������ʱ������Ϊ�������������Ӧ���µģ�����ʽΪAl2O3+6H+="2" Al3++3H2O��
��3������Ũ�ȿ�֪�����ӵ�Ũ������ͬ�ģ����Ԣٲ���ȷ����Ӧ�ж����������ӣ��ܲ���ȷ�����������������Dz�ͬ�ģ����ᷴӦ�죬��˵��Cl���д����ã�������SO42�����軯���ã���ѡ�ڢܡ�
��4��������������Է�Ӧ���ʵ�Ӱ�졣һ������Ӧ���Ũ�ȡ������¶ȡ����������������ԭ��ط�Ӧ��������Ӧ��ĽӴ�����ȶ��ܼӿ췴Ӧ���ʡ�Ũ����������ܲ����������ܲ���ȷ������ϡ�ͽ���������Ũ�ȣ���Ӧ���ʽ��͡���������Һ�У�������ܺ�����Ӧ��������������NO�����������������Ԣ���ȷ��
(2)���ǻ��õĽ������ױ���������һ�����ܵ�����Ĥ�����Կ�ʼû��������ʱ������Ϊ�������������Ӧ���µģ�����ʽΪAl2O3+6H+="2" Al3++3H2O��
��3������Ũ�ȿ�֪�����ӵ�Ũ������ͬ�ģ����Ԣٲ���ȷ����Ӧ�ж����������ӣ��ܲ���ȷ�����������������Dz�ͬ�ģ����ᷴӦ�죬��˵��Cl���д����ã�������SO42�����軯���ã���ѡ�ڢܡ�
��4��������������Է�Ӧ���ʵ�Ӱ�졣һ������Ӧ���Ũ�ȡ������¶ȡ����������������ԭ��ط�Ӧ��������Ӧ��ĽӴ�����ȶ��ܼӿ췴Ӧ���ʡ�Ũ����������ܲ����������ܲ���ȷ������ϡ�ͽ���������Ũ�ȣ���Ӧ���ʽ��͡���������Һ�У�������ܺ�����Ӧ��������������NO�����������������Ԣ���ȷ��

��ϰ��ϵ�д�
�����Ŀ