��Ŀ����
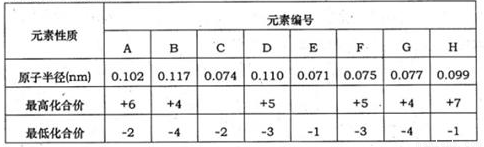
�±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ���Ӧ�⻯��е������

��֪����A��D���γɻ�����AD2��AD3���������Ʊ�ǿ��ף���B��D���γɻ�����BD��BD2���������Ʊ�ǿ���ҡ�
��ش�
��1���������ڵ�������Ԫ�ص���_________���ñ���Ԫ�ر����д����
��2����֪H���⻯��a g��ȫȼ������Һ̬ˮ����bkJ��д�����⻯��ȼ���ȵ��Ȼ�ѧ����ʽ��
________________________
��3���ɱ���DԪ�غ���Ԫ�ص�ԭ�Ӱ�1:1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬д�������������Ը�����ط�Ӧ�����ӷ���ʽ_________________��
��4���������ΪADG2��������ˮ�л�ǿ��ˮ�⣬����ʹƷ����Һ��ɫ����ɫ�����һ��ǿ�ᡣ�÷�Ӧ�Ļ�ѧ����ʽ�ǣ�__________________��
��5����ҵ����ǿ����ʱ���ô�����Һ����β�����÷�Ӧ���£�BD+BD2+Na2CO3=2_________+CO2
������ij�εĻ�ѧʽӦΪ_________
��6�������һ��ʵ�鷽����ʹ��ͭ��ϡ��ǿ���Ӧ���õ���ɫ��Һ���������ڷ����л��ʵ��װ��ͼ�������Ҫ��˵�����ɡ�
��ش�
��1���������ڵ�������Ԫ�ص���_________���ñ���Ԫ�ر����д����
��2����֪H���⻯��a g��ȫȼ������Һ̬ˮ����bkJ��д�����⻯��ȼ���ȵ��Ȼ�ѧ����ʽ��
________________________
��3���ɱ���DԪ�غ���Ԫ�ص�ԭ�Ӱ�1:1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬д�������������Ը�����ط�Ӧ�����ӷ���ʽ_________________��
��4���������ΪADG2��������ˮ�л�ǿ��ˮ�⣬����ʹƷ����Һ��ɫ����ɫ�����һ��ǿ�ᡣ�÷�Ӧ�Ļ�ѧ����ʽ�ǣ�__________________��
��5����ҵ����ǿ����ʱ���ô�����Һ����β�����÷�Ӧ���£�BD+BD2+Na2CO3=2_________+CO2
������ij�εĻ�ѧʽӦΪ_________
��6�������һ��ʵ�鷽����ʹ��ͭ��ϡ��ǿ���Ӧ���õ���ɫ��Һ���������ڷ����л��ʵ��װ��ͼ�������Ҫ��˵�����ɡ�

��1��ACEG
��2��CH4(g)+2O2(g)=CO2(g)+2H2O(l)����H=-16b/a KJ/mol
��3��5H2O2+2MnO4-+6H+=8H2O+2Mn2++5O2��
��4��SOCl2+H2O��SO2+2HCl
��5��NaNO2
��6��
��2��CH4(g)+2O2(g)=CO2(g)+2H2O(l)����H=-16b/a KJ/mol
��3��5H2O2+2MnO4-+6H+=8H2O+2Mn2++5O2��
��4��SOCl2+H2O��SO2+2HCl
��5��NaNO2
��6��


��ϰ��ϵ�д�
 ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
�����Ŀ


 �±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ�����Ӧԭ�Ӱ뾶�����ݣ���ش��������⣺
�±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ�����Ӧԭ�Ӱ뾶�����ݣ���ش��������⣺