��Ŀ����
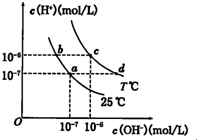
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-������ͼ��ʾ��ϵ������˵����ȷ���ǣ�������


| A��a���Ӧ����Һ��c���Ӧ����ҺpHֵ��С��pH��c����pH��a�� | B��d���Ӧ����Һ�д������ڣ�K+��Ba2+��NO3-��I- | C��25��ʱ��Ka��HF��=3.6��10-4��Ka��CH3COOH��=1.75��10-5��0.1mol/L��NaF��Һ��0.1mol/L ��CH3COOK��Һ��ȣ�c��Na+��-c��F-����c��K+��-c��CH3COO-�� | D����b���Ӧ����Һ��ֻ��NaHA������Һ������Ũ�ȴ�С��c��HA-����c��H2A����c��H+����c��A2-�� |
������A��������Ũ��Խ��pHԽС��
B��d����Һ��c��H+����c��OH-������Һ�ʼ��ԣ�������������������Ӻ͵����Ӳ���Ӧ��
C����ͬ�¶��£�F-ˮ��̶�С��CH3COO-������ͬŨ�ȵ�NaF��Һ����С��CH3COOK�����ݵ���غ���㣻
D��b����Һc��H+����c��OH-������Һ�����ԣ�HA-�ĵ���̶ȴ���ˮ��̶ȣ�
B��d����Һ��c��H+����c��OH-������Һ�ʼ��ԣ�������������������Ӻ͵����Ӳ���Ӧ��
C����ͬ�¶��£�F-ˮ��̶�С��CH3COO-������ͬŨ�ȵ�NaF��Һ����С��CH3COOK�����ݵ���غ���㣻
D��b����Һc��H+����c��OH-������Һ�����ԣ�HA-�ĵ���̶ȴ���ˮ��̶ȣ�
����⣺A��������Ũ��Խ��pHԽС����a���Ӧ����Һ��c���Ӧ����ҺpHֵ��С��pH��c����pH��a������A����
B��d����Һ��c��H+����c��OH-������Һ�ʼ��ԣ�������������������Ӻ͵����Ӳ���Ӧ�������⼸�������ܹ��棬��B��ȷ��
C����ͬ�¶��£�F-ˮ��̶�С��CH3COO-������ͬŨ�ȵ�NaF��Һ����С��CH3COOK�����ݵ���غ��c��Na+��-c��F-��=c��OH-��-c��H+����c��K+��-c��CH3COO-��=c��OH-��-c��H+����CH3COOK��Һ���Դ���NaF������c��Na+��-c��F-����c��K+��-c��CH3COO-������C����
D��b����Һc��H+����c��OH-������Һ�����ԣ�HA-�ĵ���̶ȴ���ˮ��̶ȣ�����c��H2A����c��A2-������D����
��ѡB��
B��d����Һ��c��H+����c��OH-������Һ�ʼ��ԣ�������������������Ӻ͵����Ӳ���Ӧ�������⼸�������ܹ��棬��B��ȷ��
C����ͬ�¶��£�F-ˮ��̶�С��CH3COO-������ͬŨ�ȵ�NaF��Һ����С��CH3COOK�����ݵ���غ��c��Na+��-c��F-��=c��OH-��-c��H+����c��K+��-c��CH3COO-��=c��OH-��-c��H+����CH3COOK��Һ���Դ���NaF������c��Na+��-c��F-����c��K+��-c��CH3COO-������C����
D��b����Һc��H+����c��OH-������Һ�����ԣ�HA-�ĵ���̶ȴ���ˮ��̶ȣ�����c��H2A����c��A2-������D����
��ѡB��
���������⿼�������ӻ��������й�֪ʶ����ȷ���߱仯���Ƽ������ӡ�����������֮��Ĺ�ϵ�ǽⱾ��ؼ������ݸ�������Һ������ٽ�ϵ���غ�����������״�ѡ����B��ע�⣺���������£���������Ӿ���ǿ�����ԣ�����������ܺ͵����ӷ���������ԭ��Ӧ�����Ի���������¶��߲���Ӧ��Ϊ�״��㣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
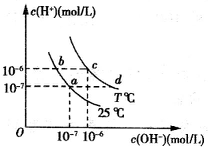
 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH+������ͼ��ʾ�Ĺ�ϵ������˵���в���ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH+������ͼ��ʾ�Ĺ�ϵ������˵���в���ȷ���ǣ�������| A��ͼ��T��25 | B��b����Һc��H+��һ����a��� | C��c���Ӧ����Һ�п��ܴ�������Al3+��Cl- | D��d���Ӧ����Һ�ʼ��� |
 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-���Ĺ�ϵ��ͼ��ʾ�������й�˵������ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-���Ĺ�ϵ��ͼ��ʾ�������й�˵������ȷ���ǣ�������| A������a�㵽c�㣬�ɲ�����ˮ�м�����ķ��� | B��b���Ӧ�Ĵ�������ˮ�����c��H+��=10-6mol/L | C��c���Ӧ��Һ��Kw����d���Ӧ��Һ��Kw | D��T��ʱ��0.05 mol?L-1��Ba��OH��2��Һ��pH=11 |
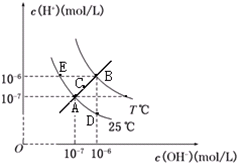 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-����ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-����ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A��ͼ�����Kw��Ĺ�ϵ��B��C��A=D=E | B��E���Ӧ��ˮ��Һ�У�������NH4+��Ba2+��Cl-��I-����ͬʱ���� | C��������B��ʱ����pH=2��������Һ��pH=10��KOH��Һ�������ϣ�������Һ������ | D����0.1 mol/L��NaHA��Һˮ��Һ��c��H+����c��OH-����ϵ��ͼD����ʾ������Һ���У�c��HA-����c��OH-����c��A2-����c��H2A�� |
 �ڲ�ͬ�¶��µ�ˮ��Һ������Ũ��������ͼ��ʾ������˵������ȷ���ǣ�������
�ڲ�ͬ�¶��µ�ˮ��Һ������Ũ��������ͼ��ʾ������˵������ȷ���ǣ������� �ڲ�ͬ�¶��µ�ˮ��Һ��c��H+��=10x mol/L��c��OH-��=10y mol/L��x��y�Ĺ�ϵ��ͼ��ʾ����ش��������⣺
�ڲ�ͬ�¶��µ�ˮ��Һ��c��H+��=10x mol/L��c��OH-��=10y mol/L��x��y�Ĺ�ϵ��ͼ��ʾ����ش��������⣺