��Ŀ����
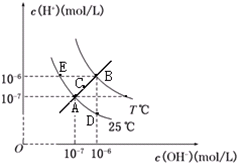 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-����ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-����ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A��ͼ�����Kw��Ĺ�ϵ��B��C��A=D=E | B��E���Ӧ��ˮ��Һ�У�������NH4+��Ba2+��Cl-��I-����ͬʱ���� | C��������B��ʱ����pH=2��������Һ��pH=10��KOH��Һ�������ϣ�������Һ������ | D����0.1 mol/L��NaHA��Һˮ��Һ��c��H+����c��OH-����ϵ��ͼD����ʾ������Һ���У�c��HA-����c��OH-����c��A2-����c��H2A�� |
��������ͼ���֪��A��E��D��Ϊ25��ʱ�������ϣ�ˮ��Kwֻ���¶��йأ��¶���ͬʱKw��ͬ���¶����ߣ��ٽ�ˮ�ĵ��룬Kw����ˮ�����ӻ�ֻ���¶��йأ�����Һ�������أ�����Ϊ��ˮ��Ҳ����Ϊ�ᡢ�����Һ��
����⣺A����ͼ���֪��A��E��D��Ϊ25��ʱ�������ϣ�ˮ��Kwֻ���¶��йأ��¶���ͬʱKw��ͬ���¶����ߣ��ٽ�ˮ�ĵ��룬Kw������B��A=D=E����C��c��OH-����c��H+����֪��C���Kw����A��С��B������B��C��A=D=E����A��ȷ��
B��E��c��OH-����c��H+������Һ�����ԣ����������£�NH4+��Ba2+��Cl-��I-û�г��������塢������ʡ�������ԭ��Ӧ�����������ܹ��棬��B��ȷ��
C��B��ʱ��Kw=1��10-12��pH=2��������c��H+��=0.01mol/L����pH=10��KOH��Һ��c��OH-��=0.01mol/L���������Ϻ���Һ�����ԣ���C��ȷ��
D��D����Һ��c��OH-����c��H+������Һ�ʼ��ԣ�˵��HA-��ˮ��̶ȴ��ڵ���̶ȣ�����c��A2-����c��H2A������D����
��ѡD��
B��E��c��OH-����c��H+������Һ�����ԣ����������£�NH4+��Ba2+��Cl-��I-û�г��������塢������ʡ�������ԭ��Ӧ�����������ܹ��棬��B��ȷ��
C��B��ʱ��Kw=1��10-12��pH=2��������c��H+��=0.01mol/L����pH=10��KOH��Һ��c��OH-��=0.01mol/L���������Ϻ���Һ�����ԣ���C��ȷ��
D��D����Һ��c��OH-����c��H+������Һ�ʼ��ԣ�˵��HA-��ˮ��̶ȴ��ڵ���̶ȣ�����c��A2-����c��H2A������D����
��ѡD��
���������⿼��������ʵĵ��룬��Ŀ�Ѷ��еȣ�ע�����ͼ����ˮ�����ӻ�ֻ���¶ȵ�Ӱ�죮

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д�
�����Ŀ
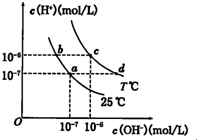 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH+������ͼ��ʾ�Ĺ�ϵ������˵���в���ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH+������ͼ��ʾ�Ĺ�ϵ������˵���в���ȷ���ǣ�������| A��ͼ��T��25 | B��b����Һc��H+��һ����a��� | C��c���Ӧ����Һ�п��ܴ�������Al3+��Cl- | D��d���Ӧ����Һ�ʼ��� |
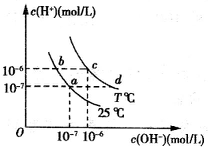 �ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-���Ĺ�ϵ��ͼ��ʾ�������й�˵������ȷ���ǣ�������
�ڲ�ͬ�¶��£�ˮ��Һ��c��H+����c��OH-���Ĺ�ϵ��ͼ��ʾ�������й�˵������ȷ���ǣ�������| A������a�㵽c�㣬�ɲ�����ˮ�м�����ķ��� | B��b���Ӧ�Ĵ�������ˮ�����c��H+��=10-6mol/L | C��c���Ӧ��Һ��Kw����d���Ӧ��Һ��Kw | D��T��ʱ��0.05 mol?L-1��Ba��OH��2��Һ��pH=11 |
 �ڲ�ͬ�¶��µ�ˮ��Һ������Ũ��������ͼ��ʾ������˵������ȷ���ǣ�������
�ڲ�ͬ�¶��µ�ˮ��Һ������Ũ��������ͼ��ʾ������˵������ȷ���ǣ������� �ڲ�ͬ�¶��µ�ˮ��Һ��c��H+��=10x mol/L��c��OH-��=10y mol/L��x��y�Ĺ�ϵ��ͼ��ʾ����ش��������⣺
�ڲ�ͬ�¶��µ�ˮ��Һ��c��H+��=10x mol/L��c��OH-��=10y mol/L��x��y�Ĺ�ϵ��ͼ��ʾ����ش��������⣺