��Ŀ����
 ����ͼ��ʾ��װ�ý��е�⣮ͨ��һ���������ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ��
����ͼ��ʾ��װ�ý��е�⣮ͨ��һ���������ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ����1��A��Fe�缫���������ĵ缫����ʽΪ
4AgNO3+2H2O
4Ag+O2+4HNO3
| ||
4AgNO3+2H2O
4Ag+O2+4HNO3
��
| ||
��2����B�й۲쵽��������
ͭƬ�ܽ⣬�������ɡ���ɫ����
ͭƬ�ܽ⣬�������ɡ���ɫ����
����3�������£����ӵ�ʼ��������A��Bװ���й��ռ�������0.168L�����������������������������Ӧ���������ⶨ����A����Һ���ǡΪ1000mL����A��Һ��pH��
2
2
����������1��ͨ��һ���������ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ��˵��C������������E�Ǹ�����F��������������������˿�������������������Һʱ�������������ӷŵ磬���������������ӷŵ磻
��2�������������Һ��ͭ����������˿��������������ͭʧ���ӷ���������Ӧ�������������ӷŵ磻
��3������ת�Ƶ����غ���н��
��2�������������Һ��ͭ����������˿��������������ͭʧ���ӷ���������Ӧ�������������ӷŵ磻
��3������ת�Ƶ����غ���н��
����⣺��1��ͨ��һ���������ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ��˵��C������������E�Ǹ�����F��������������������˿�������������������Һʱ�������������ӷŵ磬���������������ӷŵ磬
��ⷽ��ʽΪ4AgNO3+2H2O
4Ag+O2+4HNO3��
�ʴ�Ϊ��4AgNO3+2H2O
4Ag+O2+4HNO3��
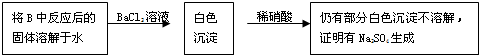
��2��B��ʢ�б���Na2SO4��Һ��CuΪ��������������ͭ���ӣ�Cu����Χ����ɫ������������������Һ����������ͭ������
�ʴ�Ϊ��ͭƬ�ܽ⣬�������ɡ���ɫ������
��4��A��Bװ���й��ռ�����״���µ�����0.168L��Ӧ�ֱ�Ϊ������������������Ϊ0.168L��
=0.056L��n��02��=
=0.0025mol��ת�Ƶ���0.01mol��
A��ʢ��AgNO3��Һ�����ʱ�������ᣬn��H+��=n��e-��=0.01mol����c��H+��=
=0.01mol/L����pH=2��
�ʴ�Ϊ��2��
��ⷽ��ʽΪ4AgNO3+2H2O
| ||
�ʴ�Ϊ��4AgNO3+2H2O
| ||
��2��B��ʢ�б���Na2SO4��Һ��CuΪ��������������ͭ���ӣ�Cu����Χ����ɫ������������������Һ����������ͭ������
�ʴ�Ϊ��ͭƬ�ܽ⣬�������ɡ���ɫ������
��4��A��Bװ���й��ռ�����״���µ�����0.168L��Ӧ�ֱ�Ϊ������������������Ϊ0.168L��
| 1 |
| 3 |
| 0.056L |
| 22.4L/mol |
A��ʢ��AgNO3��Һ�����ʱ�������ᣬn��H+��=n��e-��=0.01mol����c��H+��=
| 0.1mol |
| 1L |
�ʴ�Ϊ��2��
�����������ۺϿ�����ԭ���������ڿ���ѧ���ۺ����õ��֪ʶ����������Ŀ�ѶȽϴ�ע����յ����ɣ�����ȷ�жϵ缫����ʽ��

��ϰ��ϵ�д�
�����Ŀ
 ijͬѧ����ͼ��ʾ��װ�ý���ͭ��Ũ���ᷴӦ��ʵ�飮��ش����⣮
ijͬѧ����ͼ��ʾ��װ�ý���ͭ��Ũ���ᷴӦ��ʵ�飮��ش����⣮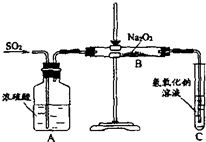
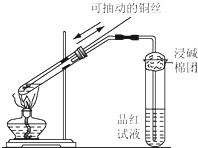
 ij����С��������ͼ��ʾ��װ�ý���ʵ�飮���мס��ҡ�����λͬѧ�ֱ�ѡ�������µ缫���Ϻ͵������Һ��
ij����С��������ͼ��ʾ��װ�ý���ʵ�飮���мס��ҡ�����λͬѧ�ֱ�ѡ�������µ缫���Ϻ͵������Һ�� ����ͼ��ʾ��װ�ý��е�⣮A��ʢ��AgNO3��Һ��B��ʢ�б���Na2SO4��Һͨ��һ���������ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ����
����ͼ��ʾ��װ�ý��е�⣮A��ʢ��AgNO3��Һ��B��ʢ�б���Na2SO4��Һͨ��һ���������ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ����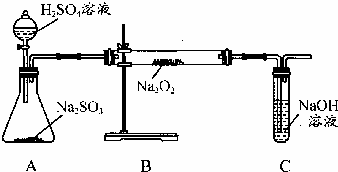 ij��ѧѧϰС���ͬѧΪ̽�������������������ķ�Ӧ������ͼ��ʾ��װ�ý���ʵ�飮ͨ����������������ǵ�ľ�������Թ�C��ľ����ȼ����ش���������
ij��ѧѧϰС���ͬѧΪ̽�������������������ķ�Ӧ������ͼ��ʾ��װ�ý���ʵ�飮ͨ����������������ǵ�ľ�������Թ�C��ľ����ȼ����ش���������