��Ŀ����
������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⣺
(1)��¯ұ�������У������ڴ���Ӧ���в���ˮú��(CO��H2)��ԭ���������йط�ӦΪ��CH4(g)��CO2(g)=2CO(g)��2H2(g)����H��260 kJ��mol��1
��֪��2CO(g)��O2(g)=2CO2(g)����H����566 kJ��mol��1
��CH4��O2��Ӧ����CO��H2���Ȼ�ѧ����ʽΪ_____________________��
(2)����ͼ��ʾ��װ�â�Ϊ����ȼ�ϵ��(�������ҺΪKOH��Һ)��ͨ��װ�â�ʵ�������϶�ͭ��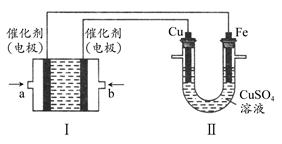
��a��Ӧͨ��________(�CH4����O2��)��b���缫�Ϸ����ĵ缫��Ӧʽ��________��
�ڵ�ƽ�����װ�â�����Һ��pH________(��д�������С�����䡱����ͬ)��װ�â���Cu2�������ʵ���Ũ��________��
�۵�ƽ�����װ�â���Һ�е������ӳ���OH���������________(����ˮ��)��
���ڴ˹���������ȫ��Ӧ��װ�â������������仯12.8 g����װ�â������������ļ���________L(��״����)��
(1)2CH4(g)��O2(g)=2CO(g)��4H2(g)����H����46 kJ��mol��1
(2)��CH4��O2��2H2O��4e��=4OH�����ڱ�С�����䡡��CO32������0.56
����

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�̼���仯�����й㷺����;��
(1)��ˮ����ͨ�����ȵ�̼���ɲ���ˮú����ӦΪ
C(s)��H2O(g) CO(g)��H2(g)����H����131.3 kJ��mol��1��
CO(g)��H2(g)����H����131.3 kJ��mol��1��
���Ϸ�Ӧ�ﵽƽ������������������£����´�ʩ���������H2O��ƽ��ת���ʵ���________��(�����)
| A�������¶� | B������̼������ | C��������� | D����CO���ռ���ȥCO |
 2CO(g)����H����172.5 kJ��mol��1����CO(g)��H2O(g)
2CO(g)����H����172.5 kJ��mol��1����CO(g)��H2O(g) CO2(g)��H2(g)���ʱ䦤H��________��
CO2(g)��H2(g)���ʱ䦤H��________��(3)CO��H2��һ�������¿ɷ�Ӧ���ɼ״���CO(g)��2H2(g)
 CH3OH(g)���״���һ��ȼ�ϣ������ü״����һ��ȼ�ϵ�أ���ϡ�������������Һ�����ʯī���缫���õ�ظ�����ӦʽΪ______________________________________��
CH3OH(g)���״���һ��ȼ�ϣ������ü״����һ��ȼ�ϵ�أ���ϡ�������������Һ�����ʯī���缫���õ�ظ�����ӦʽΪ______________________________________�����øõ���ṩ�ĵ��ܵ��60 mL NaCl��Һ������0.01 mol CH3OH��ȫ�ŵ磬NaCl�������ҵ�������Cl2ȫ���ݳ������ǰ�������Һ����ı仯�����������������Һ��pH��________��
(4)��һ������CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2.0 L�ĺ����ܱ������У��������·�Ӧ��CO(g)��H2O(g)
 CO2(g)��H2(g)���õ��������ݣ�
CO2(g)��H2(g)���õ��������ݣ�| �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ���� ��ʱ��/min | ||
| H2O | CO | H2 | CO | | |
| 900 | 1.0 | 2.0 | 0.4 | 1.6 | 3.0 |
ͨ����������÷�Ӧ��ƽ�ⳣ��(���������λ��Ч����)________���ı䷴Ӧ��ijһ��������Ӧ���е�t minʱ����û��������CO2�����ʵ���Ϊ0.6 mol������200 mL 5 mol/L��NaOH��Һ������ȫ���գ���Ӧ�����ӷ���ʽΪ(��һ�����ӷ���ʽ��ʾ)_________________________
(5)��ҵ�����ǰ�ˮú���еĻ�����徭���������õĽϴ�H2���ںϳɰ����ϳɰ���Ӧԭ��ΪN2(g)��3H2(g)
 2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��
2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��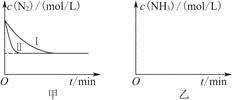
��ش��������⣺
����ʵ���Ƚϣ�ʵ���ı������Ϊ________________________________��
��ʵ����ʵ�����¶�Ҫ�ߣ�����������ͬ������ͼ���л���ʵ����ʵ�����NH3Ũ����ʱ��仯��ʾ��ͼ��
�±��е��������ƻ�1 mol�����еĻ�ѧ�������ĵ�����(kJ):
| ���� | Cl2 | Br2 | I2 | HCl | HBr | HI | H2 |
| ����/kJ | 243 | 193 | 151 | 432 | 366 | 298 | 436 |
�����������ݻش�(1)~(5)�⡣
(1)�������ʱ������е�������͵�������������
A.H2 B.Cl2 C.Br2 D.I2
(2)�����⻯����,���ȶ���������������
A.HCl����������B.HBr����������C.HI
(3)X2+H2
 2HX(X����Cl��Br��I )�ķ�Ӧ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ?��:��______________��
2HX(X����Cl��Br��I )�ķ�Ӧ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ?��:��______________�� (4)��ͬ������,X2(X����Cl��Br��I)�ֱ���������Ӧ,�����ĵ����ʵ���������ʱ,�ų������յ�����������������������
(5)�����ϱ��е�����,������ȷ�ش������(4)��?
��:����������,��ĸ�������______________________��
 CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��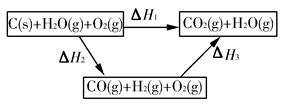
 CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��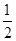 O2(g)=H2O(g)����H����242.0 kJ��mol��1
O2(g)=H2O(g)����H����242.0 kJ��mol��1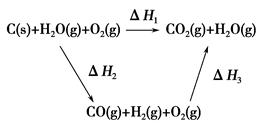
 N2(g)��2CO2(g) ��H=��a kJ��mol-1��
N2(g)��2CO2(g) ��H=��a kJ��mol-1��
 O2��g��=
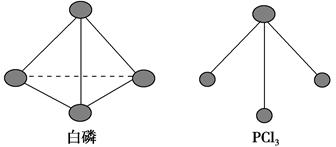
O2��g��=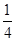 P4O10��s����H2����738.5 kJ��mol��1������¶��°���ת��Ϊ�����Ȼ�ѧ����ʽΪ________________��
P4O10��s����H2����738.5 kJ��mol��1������¶��°���ת��Ϊ�����Ȼ�ѧ����ʽΪ________________��