��Ŀ����
�������ߴ����Ŵ����Ļ�ѧ���ʣ��ش��������⣺
��1��̼�������DZ��Ƹ�㷢�ͷ۵���Ҫ�ɷ�֮һ����ѧʽΪ ��
��2��ij������������Cl��O����Ԫ����ɣ�ÿһ����������1��Clԭ�ӡ�2��Oԭ�ӣ����������Ļ�ѧʽ�� ��
��3��ʳ���к��д��ᣨCH3COOH����������ˮ��Һ���ܽ����CH3COO- �� �������ӷ��ţ���
��4������ᣨHF����Һ�������ڲ�����̣�ԭ����������벣���еĶ������跴Ӧ�������ķ��������壨SiF4����ˮ����ѧ����ʽΪ ��
��1��̼�������DZ��Ƹ�㷢�ͷ۵���Ҫ�ɷ�֮һ����ѧʽΪ
��2��ij������������Cl��O����Ԫ����ɣ�ÿһ����������1��Clԭ�ӡ�2��Oԭ�ӣ����������Ļ�ѧʽ��
��3��ʳ���к��д��ᣨCH3COOH����������ˮ��Һ���ܽ����CH3COO- ��
��4������ᣨHF����Һ�������ڲ�����̣�ԭ����������벣���еĶ������跴Ӧ�������ķ��������壨SiF4����ˮ����ѧ����ʽΪ
���㣺��ѧʽ����д������,��ѧ���ż�����Χ���ֵ�����,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺��ѧ����������غ㶨��
��������1��̼�������DZ��Ƹ�㷢�ͷ۵���Ҫ�ɷ�֮һ����Ԫ����+1�ۣ�̼�������-1�ۣ��ݴ˽��з������
��2�������⣬ij������������Cl��O����Ԫ����ɣ�ÿһ����������1��Clԭ�ӡ�2��Oԭ�ӣ��ݴ˽��з������
��3��ʳ���к��д��ᣨCH3COOH����������ˮ��Һ���ܽ����CH3COO- �������ӣ�
��4��������벣���еĶ������跴Ӧ�������ķ��������壨SiF4����ˮ��д����Ӧ�Ļ�ѧ����ʽ���ɣ�
��2�������⣬ij������������Cl��O����Ԫ����ɣ�ÿһ����������1��Clԭ�ӡ�2��Oԭ�ӣ��ݴ˽��з������
��3��ʳ���к��д��ᣨCH3COOH����������ˮ��Һ���ܽ����CH3COO- �������ӣ�
��4��������벣���еĶ������跴Ӧ�������ķ��������壨SiF4����ˮ��д����Ӧ�Ļ�ѧ����ʽ���ɣ�
����⣺��1��̼�������DZ��Ƹ�㷢�ͷ۵���Ҫ�ɷ�֮һ����Ԫ����+1�ۣ�̼�������-1�ۣ���ѧʽΪNaHCO3��
��2��ij������������Cl��O����Ԫ����ɣ�ÿһ����������1��Clԭ�ӡ�2��Oԭ�ӣ����������Ļ�ѧʽ��ClO2��
��3��ʳ���к��д��ᣨCH3COOH����������ˮ��Һ���ܽ����CH3COO- �������ӣ������ӵ����ӷ���Ϊ��H+��
��4��������벣���еĶ������跴Ӧ�������ķ��������壨SiF4����ˮ����Ӧ�Ļ�ѧ����ʽΪ��SiO2+4HF=SiF4��+2H2O��
�ʴ�Ϊ����1��NaHCO3����2��ClO2����3��H+����4��SiO2+4HF=SiF4��+2H2O��
��2��ij������������Cl��O����Ԫ����ɣ�ÿһ����������1��Clԭ�ӡ�2��Oԭ�ӣ����������Ļ�ѧʽ��ClO2��
��3��ʳ���к��д��ᣨCH3COOH����������ˮ��Һ���ܽ����CH3COO- �������ӣ������ӵ����ӷ���Ϊ��H+��
��4��������벣���еĶ������跴Ӧ�������ķ��������壨SiF4����ˮ����Ӧ�Ļ�ѧ����ʽΪ��SiO2+4HF=SiF4��+2H2O��
�ʴ�Ϊ����1��NaHCO3����2��ClO2����3��H+����4��SiO2+4HF=SiF4��+2H2O��
�����������ѶȲ�����Ҫ����ͬѧ�ǶԳ�����ѧ�����ѧʽ�����ӷ��š���ѧ����ʽ�ȣ�����д������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ij���ӵĽṹʾ��ͼ��ͼ�����и�����������Ӵ���ͬһ�����һ�ѧ�������Ƶ��ǣ�������
ij���ӵĽṹʾ��ͼ��ͼ�����и�����������Ӵ���ͬһ�����һ�ѧ�������Ƶ��ǣ�������




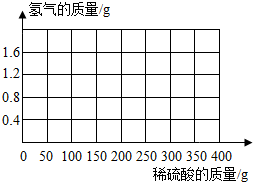 Ϊ�˲ⶨij�ֻ�ͭ��ͭ��п�ĺϽ𣩵���ɣ�ȡ�û�ͭ��Ʒ��м100g����400gϡ�����4�μ��뵽����Ʒ�У�������ݼ�¼�����
Ϊ�˲ⶨij�ֻ�ͭ��ͭ��п�ĺϽ𣩵���ɣ�ȡ�û�ͭ��Ʒ��м100g����400gϡ�����4�μ��뵽����Ʒ�У�������ݼ�¼�����

