��Ŀ����
���ȼҵ�����ҹ�Ŀǰ��ѧ��ҵ����Ҫ֧��֮һ��������Ҫԭ���ǵ�ⱥ��ʳ��ˮ���仯ѧ����ʽΪxNaCl+2H2O
xNaOH+H2��+Cl2������20��ʱ��ȡ100g����NaCl��Һ���е�⣬һ��ʱ����ò���������Cl2��������Ϊ7.1g����֪��20��ʱ��NaC1���ܽ��Ϊ36g��
�����������ش�
��1�����������غ㶨�ɿ�֪������Ӧ��x= ��
��2��ͨ�����㣬����������ͬʱ����H2������Ϊ g��
��3���������ʣ����Һ��NaC1������������д����ϸ�ļ�����̣������ȷ��0.1%����
| ||
�����������ش�
��1�����������غ㶨�ɿ�֪������Ӧ��x=
��2��ͨ�����㣬����������ͬʱ����H2������Ϊ
��3���������ʣ����Һ��NaC1������������д����ϸ�ļ�����̣������ȷ��0.1%����
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���,�����غ㶨�ɼ���Ӧ��
ר�⣺�������������뻯ѧ����ʽ���ϵļ���
��������1�����������غ㶨�ɷ�����
��2�������������������û�ѧ����ʽ����������������
��3�����ݻ�ѧ����ʽ���������������������Ӧ���Ȼ��Ƶ��������������ʣ���Ȼ��Ƶ���������������������
��2�������������������û�ѧ����ʽ����������������
��3�����ݻ�ѧ����ʽ���������������������Ӧ���Ȼ��Ƶ��������������ʣ���Ȼ��Ƶ���������������������
����⣺��1�����������غ㶨�ɣ���Ӧǰ��ԭ�ӵĸ������䣬��x=2��
�ʴ�Ϊ��2��
������������Ϊx���μӷ�Ӧ���Ȼ��Ƶ�����Ϊy
2NaCl+2H2O
2NaOH+H2��+Cl2��
117 2 71
y x 7.1g
=
=
x=0.2g��y=11.7g
��2����������������Ϊ0.2g��
��3������ʣ����Һ��NaC1������Ϊ��100g��
��100%-11.7g=14.8g
����ʣ����Һ��NaC1������������
��100%=16.0%
�𣺵���ʣ����Һ��NaC1����������Ϊ16.0%��
�ʴ�Ϊ��2��
������������Ϊx���μӷ�Ӧ���Ȼ��Ƶ�����Ϊy
2NaCl+2H2O
| ||
117 2 71
y x 7.1g
| 117 |
| y |
| 2 |
| x |
| 71 |
| 7.1g |
x=0.2g��y=11.7g
��2����������������Ϊ0.2g��
��3������ʣ����Һ��NaC1������Ϊ��100g��
| 36g |
| 100g+36g |
����ʣ����Һ��NaC1������������
| 14.8g |
| 100g-0.2g-7.1g |
�𣺵���ʣ����Һ��NaC1����������Ϊ16.0%��
�������������ܽ������Һ�����ʵ�����������

��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ
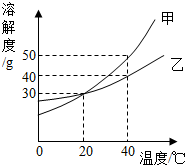 ��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�����˵����ȷ���ǣ�������
��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�����˵����ȷ���ǣ�������| A��20��ʱ���ס�����Һ�����ʵ������������ |
| B��20��ʱ����60g����100gˮ�еõ���ҺA�����ټ���100gˮ����ʹ��ҺA������������С |
| C��40��ʱ���ı�����Һ�����ʵ���������ԼΪ33.3% |
| D���ɲ��ý����¶ȵķ�����ʹ�ҵı�����Һ��Ϊ��������Һ |
��������й��Ĵ�ͳ���գ��������Ƕ���ڵ�һ����ɫ��С��������������ԭ�ϰ����ӣ����в���ʹ�õ��ǣ�������
| A������Ŵ�� |
| B������� |
| C��ǿ�������� |
| D����ȩ���ݹ�����Ҷ |
 ��ͼ��ʾ��A��H�dz��л�ѧ���������ʣ�A��B�ɷ����кͷ�Ӧ��X������ʳƷ�������YΪ���ʣ�����ͼʾ�ش��������⣺
��ͼ��ʾ��A��H�dz��л�ѧ���������ʣ�A��B�ɷ����кͷ�Ӧ��X������ʳƷ�������YΪ���ʣ�����ͼʾ�ش��������⣺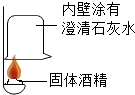 ��һ���ò��У�ͬѧ�Ƕ�ȼ�ϡ�����ƾ��������˺��棬���¶���ɷֽ����о���
��һ���ò��У�ͬѧ�Ƕ�ȼ�ϡ�����ƾ��������˺��棬���¶���ɷֽ����о���
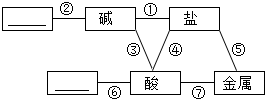 ������ѧϰ������Ҫ�������ܽ����ͼ��ʾ������֮��ķ�Ӧ��ϵ��ͼ�ж������ӵ����ʱ�ʾ�����Ӧ���������������пհף�
������ѧϰ������Ҫ�������ܽ����ͼ��ʾ������֮��ķ�Ӧ��ϵ��ͼ�ж������ӵ����ʱ�ʾ�����Ӧ���������������пհף�