��Ŀ����
����Cu����һ�ֽ�����������Mg��Fe��Zn�е�һ�֣��γɵķ�ĩ�������ⶨ����ɣ���������ʵ�飺ȡ�÷�ĩ16g�����ձ�����������������Ϊ14%��ϡ����280.0g��4�μ�����ձ��У���ַ�Ӧ���ʣ��Ĺ����������ݼ�¼���£�
|
ʵ����� |
1 |
2 |
3 |
4 |
|
����ϡ����������g |
70.0 |
70.0 |
70.0 |
70.0 |
|
ʣ�����������g |
13.6 |
11.2 |
8.8 |
8.4 |
���㣨Ҫ��д��������̣���
��1���÷�ĩ��Cu������������
��2���÷�ĩ����һ�ֽ���Ϊ���ֽ�����
��3����3�μ���ϡ�����ַ�Ӧ��������Һ�����ʵ�����������
��1��52.5 % ��2�֣�
��2���ý������ԭ������Ϊ 24 ���Խ���Ϊþ ��3�֣�
��3��16.6% ��3�֣�
��������
�����������1��Cu���ܺ�ϡH2SO4��Ӧ��ʣ���������ΪCu��������
�÷�ĩ��Cu���������� =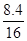 ��
100% = 52.5%
��
100% = 52.5%
��2����÷�ĩ����һ�ֽ���Ϊ M���ý������ԭ������Ϊx
M + H2SO4 = MSO4 + H2 ��
X 98
2.4 70��14%
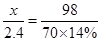
x = 24
�ý������ԭ������Ϊ 24 ���Խ���Ϊþ
��3���μӷ�Ӧ��þ������Ϊ7.6g ��������ҺΪ����þ��Һ����������þ��Һ����������������
�� þ��ϡ���ᷴӦ��������þ������Ϊy g ����������������Ϊ z g .
Mg + H2SO4 = MgSO4 + H2 ��
24 120 2
7.2 y z
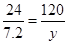
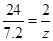
y = 36g z = 0.6g
��3�μ���ϡ�����ַ�Ӧ��������Һ�����ʵ���������= 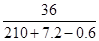 ��
100% = 16.6%
��
100% = 16.6%
�� ����
���㣺���û�ѧ����ʽ���㡣

����Cu����һ�ֽ�����������Mg��Fe��Zn�е�һ�֣��γɵķ�ĩ�������ⶨ����ɣ���������ʵ�飺ȡ�÷�ĩ16g�����ձ�����������������Ϊ14%��ϡ����280.0g��4�μ�����ձ��У���ַ�Ӧ���ʣ��Ĺ����������ݼ�¼���£�
| ���� | 1 | 2 | 3 | 4 |
| ����ϡ��������/g | 70.0 | 70.0 | 70.0 | 70.0 |
| ʣ���������/g | 13.6 | 11.2 | 8.8 | 8.4 |
��1���÷�ĩ��Cu������������
��2���÷�ĩ����һ�ֽ���Ϊ���ֽ�����
��3����3�μ���ϡ�����ַ�Ӧ��������Һ�����ʵ�����������
����Cu����һ�ֽ�����������Mg��Fe��Zn�е�һ�֣��γɵķ�ĩ�������ⶨ����ɣ���������ʵ�飺ȡ�÷�ĩ16g�����ձ�����������������Ϊ14%��ϡ����280.0g��4�μ�����ձ��У���ַ�Ӧ���ʣ��Ĺ����������ݼ�¼���£�
| ���� | 1 | 2 | 3 | 4 |
| ����ϡ����������g | 70.0 | 70.0 | 70.0 | 70.0 |
| ʣ�����������g | 13.6 | 11.2 | 8.8 | 8.4 |
���㣨Ҫ��д��������̣���
��1���÷�ĩ��Cu������������
��2���÷�ĩ����һ�ֽ���Ϊ���ֽ�����
��3����3�μ���ϡ�����ַ�Ӧ��������Һ�����ʵ�����������
