��Ŀ����
9��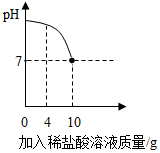 ʵ������һƿ��ǩ����ʴ�����ᣬС��Ϊ�˲ⶨ��Һ�������������������ձ���������8g 10%������������Һ��Ȼ�����ձ��еμӸ����ᣬ��Ӧ��������Һ��pH����������������ϵ��ͼ��ʾ����ش��������⣺
ʵ������һƿ��ǩ����ʴ�����ᣬС��Ϊ�˲ⶨ��Һ�������������������ձ���������8g 10%������������Һ��Ȼ�����ձ��еμӸ����ᣬ��Ӧ��������Һ��pH����������������ϵ��ͼ��ʾ����ش��������⣺��1������8g 10%������������Һ����Ҫˮ������Ϊ7.2g��
��2��������4g����ʱ���ձ�����Һ����Ԫ�ص�����Ϊ0.46g��
��3����pH=7ʱ������ϡ���������Ϊ10g��
��4�����㣺��pH=7ʱ��������Һ�����ʵ�����������д��������̣���
���� ��1����������������Һ�����������������Ƶ������������Լ���ˮ��������
��2�����۵μ����������Ϊ���٣���Һ����Ԫ�ص�������������������Һ����Ԫ�ص�������
��3������ͼ���е����ݽ��з�����
��4�����ݼ���������������Լ�����������������������
��� �⣺��1������8g10%��NaOH��Һ����Ҫˮ������Ϊ��8g����1-10%��=7.2g��
��2��������4g����ʱ���ձ�����Һ����Ԫ�ص�����Ϊ��8g��10%��$\frac{23}{40}$��100%=0.46g��
��3����pH=7ʱ������ϡ���������Ϊ10g��
��4����10gϡ�������Ȼ��������Ϊx��
NaOH+HCl�TNaCl+H2O��
40 36.5
8g��10% x
$\frac{40}{8g��10%}$=$\frac{36.5}{x}$
x=0.73g��
���������������������$\frac{0.73g}{10g}$��100%=7.3%
�𣺸������������������Ϊ7.3%��
�ʴ�Ϊ����1��7.2��
��2��0.46��
��3��10��
��4���������������������Ϊ7.3%��
���� ������Ҫ�����˻�ѧ����ʽ�ļ��㣬�ѶȲ���ע�����Ĺ淶�Ժ�ȷ�ԣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
19�������йؿ�������ɷֵ�˵���в���ȷ���ǣ�������
| A�� | ���������Ⱦָ�����У�������������̼�����������������������ͳ����� | |
| B�� | ϡ��������ͨ��ʱ�ܷ�����ͬ��ɫ�Ĺ⣬���Ƴɶ�����;�ĵ��Դ | |
| C�� | �����������ڿ�����ȼ��˵�������Ļ�ѧ���ʱȽϻ��� | |
| D�� | �����к������������ǵ�����Լռ���������78%���� |
20�����б仯��ǰ�����������仯���������ڻ�ѧ�仯���ǣ�������
| A�� | ����ͨ������ˣ���ʯ���� | |
| B�� | ������ˮ�Ʊ�����ˮ��ʳ�︯�� | |
| C�� | ú̿ȼ�գ����ͻӷ� | |
| D�� | ������̼ʹʯ��ˮ����ǣ��������� |
4�����������徭�����ã����������ڣ�������
| A�� | �������� | B�� | ���ϲ��� | C�� | �������β��� | D�� | �л��ϳɲ��� |
14���������и������ʣ�������ѡ�õ��Լ��������������ǣ�������
| A�� | ����������Һ��ϡ���ᣨ��̪��Һ�� | |
| B�� | �����ʯ��ˮ������������Һ��ϡ���ᣩ | |
| C�� | �ƾ����Ȼ�����Һ������ζ�� | |
| D�� | ������غ�����أ��۲���ɫ�� |
18��Ϊ��̽��ijϡ���������ʵ������������ס�����ͬѧ�ֱ�ȡ��������������ȵ�ϡ����100g����ͬѧ�����м�������ͭ8g����Ӧ������۲쵽����ȫ����ʧ����ͬѧ�����м�������ͭ16g����Ӧ������۲쵽������ʣ�࣮�����й�ʵ����ƶ���ȷ���ǣ�������
| A�� | ��Ӧ����ͬѧ������Һ��һ�������� | |
| B�� | ��Ӧ�����ͬѧ���õ���Һ�еμ���������������Һ��һ��������ɫ�������� | |
| C�� | ��Ӧ��ס�����ͬѧ���õ���Һ�����ʵ���������������� | |
| D�� | ԭϡ���������ʵ������������ڻ����7.3%��С��14.6% |
 ��ͼ������������ȼ�յ�ʵ�飻˼���ش����⣺
��ͼ������������ȼ�յ�ʵ�飻˼���ش����⣺