��Ŀ����
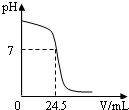
�����кͷ�Ӧ���Բⶨ������Һ�����ʵ�������������ͼΪij�βⶨ�����У���Һ��pH�����μӵ�ij����Һ����ı仯���仯�Ĺ�ϵͼ��

(1)������ͼ���߿����ж�ʵ��������________(��ᡱ��� ��ͬ)��Һ(����Һ)�еμ�_______��Һ(��Һ)��
(2)����βⶨ�У�Ϊ��ָʾ�кͷ�Ӧ�պ���ȫ���÷�̪��ָʾ���� ���ڴ�����Һ���ȵ��뼸�η�̪��Һ�����ⶨ�п���___________����֤���кͷ�Ӧ������ɡ�
(3)���ʵ����ʹ�õ��������ᣬ�����������ƣ��Ҵ���Һ�ͱ�Һ���ܶȾ���1.0g/mL�ơ����ⶨ��ȡ�ô���Һ25 mL����Һ���ʵ���������Ϊ4.5��������ͼ�����ݣ��������Һ���ʵ�����������
(2)����βⶨ�У�Ϊ��ָʾ�кͷ�Ӧ�պ���ȫ���÷�̪��ָʾ���� ���ڴ�����Һ���ȵ��뼸�η�̪��Һ�����ⶨ�п���___________����֤���кͷ�Ӧ������ɡ�
(3)���ʵ����ʹ�õ��������ᣬ�����������ƣ��Ҵ���Һ�ͱ�Һ���ܶȾ���1.0g/mL�ơ����ⶨ��ȡ�ô���Һ25 mL����Һ���ʵ���������Ϊ4.5��������ͼ�����ݣ��������Һ���ʵ�����������
(1)���
(2)����Һ�ĺ�ɫ�պ���ȥ
(3)���ĵ���H2SO4������24.5 mL��1.0g/mL=24.5 g
���ĵ���H2SO4��Һ�����ʵ�������24.5 g �� 4.5�� =1.10 g
�⣺�����Һ���ʵ���������Ϊx
��2NaOH+H2SO4 == Na2SO4+2H2O ��
x=0.036(��3.6��)
�𣺴���Һ���ʵ���������Ϊ3��6����
(2)����Һ�ĺ�ɫ�պ���ȥ
(3)���ĵ���H2SO4������24.5 mL��1.0g/mL=24.5 g
���ĵ���H2SO4��Һ�����ʵ�������24.5 g �� 4.5�� =1.10 g
�⣺�����Һ���ʵ���������Ϊx
��2NaOH+H2SO4 == Na2SO4+2H2O ��
x=0.036(��3.6��)
�𣺴���Һ���ʵ���������Ϊ3��6����

��ϰ��ϵ�д�
�����Ŀ
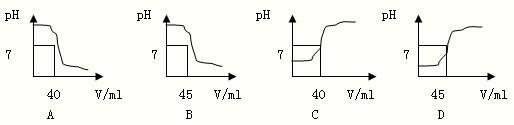
 �����кͷ�Ӧ���Բⶨ������Һ�����ʵ��������������磬��һ�����Ĵ����ᣨ��
�����кͷ�Ӧ���Բⶨ������Һ�����ʵ��������������磬��һ�����Ĵ����ᣨ��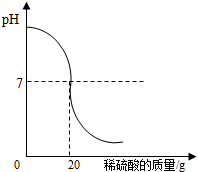 �����кͷ�Ӧ���Բⶨ������Һ�����ʵ�����������Ϊ�˲ⶨij����������Һ�����ʵ�����������ȡ����������Һ25g�������м������ʵ���������Ϊ4.9%��ϡ���ᣬ����ϡ����������pH�ı仯�������ͼ��ʾ�����㣺
�����кͷ�Ӧ���Բⶨ������Һ�����ʵ�����������Ϊ�˲ⶨij����������Һ�����ʵ�����������ȡ����������Һ25g�������м������ʵ���������Ϊ4.9%��ϡ���ᣬ����ϡ����������pH�ı仯�������ͼ��ʾ�����㣺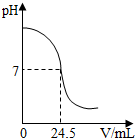 ��2013?��ɽ��һģ�������кͷ�Ӧ���Բⶨ������Һ�����ʵ�������������ͼΪij�βⶨ��������Һ��pH�����μӵ�ij����Һ����仯���仯�Ĺ�ϵͼ��
��2013?��ɽ��һģ�������кͷ�Ӧ���Բⶨ������Һ�����ʵ�������������ͼΪij�βⶨ��������Һ��pH�����μӵ�ij����Һ����仯���仯�Ĺ�ϵͼ��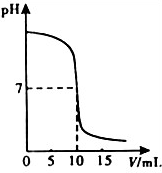 �����кͷ�Ӧ���Բⶨ������Һ�����ʵ���������������ͼΪij�βⶨ�����У���Һ��pH�����μӵ�ij����Һ����ı仯���仯�Ĺ�ϵͼ��
�����кͷ�Ӧ���Բⶨ������Һ�����ʵ���������������ͼΪij�βⶨ�����У���Һ��pH�����μӵ�ij����Һ����ı仯���仯�Ĺ�ϵͼ��