��Ŀ����
���ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õļ�Ậ��������NaCl��ij�о���ѧϰС���ȡ��NaCl��Na2CO3������25.0g���������Ƴ���Һ������������μ���������������������Ϊ7.3%��ϡ���ᣬʹ������ȫ�ų������ռ���8.8g������̼���壮�Լ��㣺��1��ԭ������Na2CO3����������������2����Ӧ�����ĵ��������������
�⣺��1����̼���Ƶ�����Ϊx��������Һ�����ʵ�����Ϊy��
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 73 44
x y 8.8g

x=21.2g

y=14.6g
 %=84.8%
%=84.8%
��ԭ������̼���Ƶ���������Ϊ84.8%��
��2����Ӧ�����ĵ������������Ϊ�� =200g �𣺷�Ӧ�����������������Ϊ200g��
=200g �𣺷�Ӧ�����������������Ϊ200g��
��������1���ɸ��ݶ�����̼���������̼���Ƶ��������ٸ��� %���ԭ������̼���Ƶ�����������
%���ԭ������̼���Ƶ�����������
��2�����ݶ�����̼���������������Һ�����ʵ��������ٸ��� ����������������
����������������
����������Ǽ�����Σ���̼���ƣ�ֻ����̼���Ƶ�ˮ��Һ�Լ��ԣ�
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 73 44
x y 8.8g

x=21.2g

y=14.6g
 %=84.8%
%=84.8%��ԭ������̼���Ƶ���������Ϊ84.8%��
��2����Ӧ�����ĵ������������Ϊ��
 =200g �𣺷�Ӧ�����������������Ϊ200g��
=200g �𣺷�Ӧ�����������������Ϊ200g����������1���ɸ��ݶ�����̼���������̼���Ƶ��������ٸ���
 %���ԭ������̼���Ƶ�����������
%���ԭ������̼���Ƶ�������������2�����ݶ�����̼���������������Һ�����ʵ��������ٸ���
 ����������������
��������������������������Ǽ�����Σ���̼���ƣ�ֻ����̼���Ƶ�ˮ��Һ�Լ��ԣ�

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�
�����Ŀ
 27����Һ�������Ϳ����о��й㷺����;����ش��������⣺
27����Һ�������Ϳ����о��й㷺����;����ش��������⣺
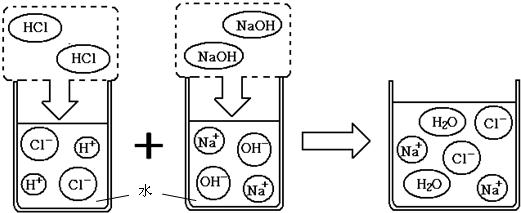
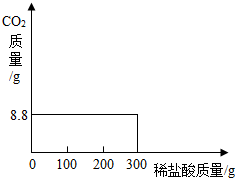 ��2003?���ݣ����ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õ�Na2CO3�Ậ��������NaCl��
��2003?���ݣ����ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õ�Na2CO3�Ậ��������NaCl��