��Ŀ����
 ��2003?���ݣ����ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õ�Na2CO3�Ậ��������NaCl��
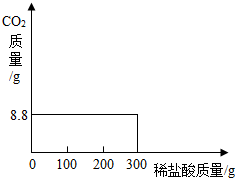
��2003?���ݣ����ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õ�Na2CO3�Ậ��������NaCl��ij�о���ѧϰС���ȡ��NaCl��Na2CO3����25.0g���������Ƴ���Һ������������μ���������7.3%��ϡ���ᣬʹ������ȫ�ų������ռ���8.8g CO2���壮
��1������ԭ������Na2CO3�����������������������������
��2���±�Ϊ�о���ѧϰС�������������ƵĻ��Һ�з�������μ����������¼�IJ������ݣ�
����������֪��Na2CO3+HCl=NaCl+NaHCO3 ��1��NaHCO3+HCl=NaCl+H2O+CO2����2��
��֪����Ӧ��1����ȫ��Ӧ��2���ſ�ʼ��
������ɱ�����δ��IJ��֣�
| ʵ����� | ÿ�β�����CO2��������g�� |
| ��һ������μ�����100g | 0 0 |
| �ڶ�������μ�����100g | 8.8 |
| ��������������100g | 0 |
��������1������̼���������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�Ļ�ѧ����ʽ���ɷų�������̼���������㷴Ӧ������̼���Ƽ�HCl����������ɸ�����ļ��㣻
��2������������������ķ�Ӧ��Ϣ����̼����������㷴Ӧ��1������Ҫ������������жϴ�ʱ��Ӧ�Ƿ�������������̼����̼�����Ƶ��������㷴Ӧ��2����Ҫ������������ų�������̼�����������жϴ�ʱ�ķ�Ӧ������Խ�һ���ƶϵ����μ�������ʱ���ܳ��ֵı仯�����
��2������������������ķ�Ӧ��Ϣ����̼����������㷴Ӧ��1������Ҫ������������жϴ�ʱ��Ӧ�Ƿ�������������̼����̼�����Ƶ��������㷴Ӧ��2����Ҫ������������ų�������̼�����������жϴ�ʱ�ķ�Ӧ������Խ�һ���ƶϵ����μ�������ʱ���ܳ��ֵı仯�����
����⣺��1����ԭ������Na2CO3������Ϊx������ϡ���������Ϊy
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 73 44
x y��7.3% 8.8g
=
=
x=21.2g y=200g
ԭ������Na2CO3����������=
��100%=84.8%
��ԭ������Na2CO3����������Ϊ84.8%�����������������Ϊ200g��
��2������Һ��21.2g̼������ɷ�Ӧ����Ҫϡ��������Ϊa������̼�����Ƶ�����Ϊb
Na2CO3+HCl=NaCl+NaHCO3
106 36.5 84
21.2g a��7.3% b
=
=
a=100g b=16.8g
��ˣ���һ�μ����100gϡ����ǡ����̼������Һ��ȫ��Ӧ�γ�̼�����ƣ��ù�����û�ж�����̼�ų���
��16.8g̼��������ɷ�Ӧ��Ҫϡ���������Ϊm���ų�������̼������Ϊn
NaHCO3+HCl=NaCl+H2O+CO2��
84 36.5 44
16.8g m��7.3% n
=
=
m=100g n=8.8g
��˵ڶ��μ���100gϡ����ʱ��ʼ�ų�������̼����ȫ��Ӧ��̼�����Ʒ�Ӧ�꣬���շų�������̼����Ϊ8.8g�����Ե����μ���ϡ����ٷ�����Ӧ��
�ʴ�Ϊ����0��
��
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 73 44
x y��7.3% 8.8g
| 106 |
| x |
| 73 |
| y��7.3% |
| 44 |
| 8.8g |
x=21.2g y=200g
ԭ������Na2CO3����������=
| 21.2g |
| 25.0g |
��ԭ������Na2CO3����������Ϊ84.8%�����������������Ϊ200g��
��2������Һ��21.2g̼������ɷ�Ӧ����Ҫϡ��������Ϊa������̼�����Ƶ�����Ϊb
Na2CO3+HCl=NaCl+NaHCO3
106 36.5 84
21.2g a��7.3% b
| 106 |
| 21.2g |
| 36.5 |
| a��7.3% |
| 84 |
| b |
a=100g b=16.8g
��ˣ���һ�μ����100gϡ����ǡ����̼������Һ��ȫ��Ӧ�γ�̼�����ƣ��ù�����û�ж�����̼�ų���
��16.8g̼��������ɷ�Ӧ��Ҫϡ���������Ϊm���ų�������̼������Ϊn
NaHCO3+HCl=NaCl+H2O+CO2��
84 36.5 44
16.8g m��7.3% n
| 84 |
| 16.8g |
| 36.5 |
| m��7.3% |
| 44 |
| n |
m=100g n=8.8g
��˵ڶ��μ���100gϡ����ʱ��ʼ�ų�������̼����ȫ��Ӧ��̼�����Ʒ�Ӧ�꣬���շų�������̼����Ϊ8.8g�����Ե����μ���ϡ����ٷ�����Ӧ��
�ʴ�Ϊ����0��
��

����������������С����ʱӦ����Դ���ǰһ��С�������û�����̼���������ᷴӦ֪ʶ��𣬺�һ��С�������Ҫ����������Ϣ������Ӧ���������ⲻ�ɻ�Ϊһ̸��

��ϰ��ϵ�д�
�����Ŀ
