��Ŀ����
4��ij������Ʒ�к��������Ȼ������ʣ�������ͼ��ʾװ�����ⶨ������Ʒ��̼���Ƶ���������������̨�����е���ͼ�о�����ȥ����ʵ�鲽�����£�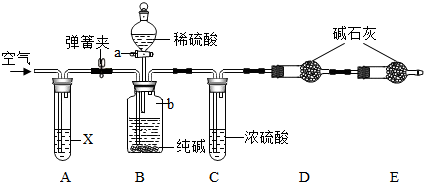
�ٰ�ͼ����װ�ã�����������ԣ�
��ȷ�Ƶ�ʢ�м�ʯ�ң������������ƺ���ʯ�ҵĻ����ĸ����D������Ϊ83.4g��
��ȷ�Ƶ�6g������Ʒ��������b�У�
�ܴ�Һ©��a����������������ϡ���ᣬ�����ٲ�������Ϊֹ��
�ݴ��ɼУ����Թ�A�л���������������ӣ�Ȼ��Ƶø����D��������Ϊ85.6g��
�Իش�
��1�����ܢ�������ʵ�����̫�죬��ᵼ�²ⶨ���ƫС���ƫ��ƫС������
��2��װ��A���Լ�XӦѡ��NaOH��Һ�������Һ��
��3����û��Cװ�ã���ᵼ�²ⶨ���ƫ���ƫ��ƫС������
��4��Eװ�õ������Ƿ�ֹ������CO2��ˮ��������D�У�
��5������ʵ���в�õ��й����ݣ������������ƷNa2CO3����������Ϊ88.3%������������һλС������
���� ��1��������Ӧ�ų������ٶȹ���Բⶨ���������Ӱ�죻
��2������װ�õ��ص㣬����װ��A����Ӧʢ�ŵ���Һ��
��3������װ��C�Բⶨ�����Ӱ�죻
��4������װ��E������װ�����������ã�
��5������ʵ��ǰ��װ��D�����仯�����ɴ������������Ʒ��̼���Ƶ������������Ʒ��̼���Ƶ�����������
��� �⣺
��1����Ӧ�����ʹ���������������̼û����ȫ��Dװ���м�ʯ�����գ����ٹ��������Ҳ��ʹװ���ڲ���������̼���ܱ�Dװ���м�ʯ����ȫ���գ�������̼����ƫС����ⶨ���ƫС��
�ʴ�Ϊ��ƫС��
��2����Ϊ�����к��ж�����̼�����Ӧ�ѹ���Ŀ����еĶ�����̼���մ���������װ��AӦ��������������Һ����������Һ��
�ʴ�Ϊ��NaOH��Һ�������Һ��
��3��Cװ���е�Ũ���������ˮ�ԣ���װ����������Bװ���ų������л��е�ˮ�֣������˴�װ�����ʹ�����е�ˮ�ֱ�Dװ���м�ʯ�����գ���ʹ�ⶨ����ƫ��
�ʴ�Ϊ��ƫ��
��4�����Dװ��ֱ��������������ͨ��������е�ˮ�Ͷ�����̼��Բⶨ�������Ӱ�죬����װ��E���������Ƿ�ֹ������ˮ�Ͷ�����̼����װ��D�У�
�ʴ�Ϊ����ֹ������CO2��ˮ��������D�У�
��5����Ӧ�зų�������̼���������=85.6g-83.4g=2.2g
��ų�2.2g������̼����̼���Ƶ�����Ϊx
Na2CO3��CO2
106 44
x 2.2g
$\frac{106}{x}=\frac{44}{2.2g}$
��֮�� x=5.3g
������ƷNa2CO3����������=$\frac{5.3g}{6g}$��100%��88.3%
�ʴ�Ϊ��88.3%��
�𰸣�
��1��ƫС
��2��NaOH��Һ�������Һ
��3��ƫ��
��4����ֹ������CO2��ˮ��������D��
��5��88.3%
���� �����ʯ�������������̼��Ӧʱ�ٶȽ�������˿��ٷų������岻����ȫ��Ӧ���ų����ⶨ������ֽϴ���

| ѡ�� | ��; | ��ѧԭ�����û�ѧ����ʽ��ʾ�� |
| A | þ�������������� | 2Mg+O2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO |
| B | ϡ�������ڳ����� | Fe+H2SO4�TFeSO4+H2�� |
| C | �������������������� | Al��OH��3+3HCl�TAlCl3+3H2O |
| D | ��Ȼ������ȼ�� | CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ��ͼΪijԪ��һ�����ӵĽṹʾ��ͼ������˵��������ǣ�������
��ͼΪijԪ��һ�����ӵĽṹʾ��ͼ������˵��������ǣ�������| A�� | ��������ԭ�ӵĽṹʾ��ͼ | B�� | �����ӵĺ˵����Ϊ1 | ||
| C�� | ��Ԫ�����ڽ���Ԫ�� | D�� | �������ڻ�ѧ��Ӧ������ʧȥ���� |
| A�� | �� | B�� | �� | C�� | �� | D�� | ���� |
| A�� | �кͷ�Ӧ�����κ�ˮ���������κ�ˮ�ķ�Ӧһ�����кͷ�Ӧ | |
| B�� | ������ֻ����һ��Ԫ�أ���ֻ����һ��Ԫ�ص�����һ���ǵ��� | |
| C�� | ���������ɲ�ͬԪ����ɵĴ�������ɲ�ͬ��Ԫ����ɵĴ�����һ���ǻ����� | |
| D�� | ���ý�������ϡ���ᷴӦ�ų����壬������ϡ���ᷴӦ�ų����������һ���ǻ��ý��� |
 ��ͼ��A��B�ɷ����кͷ�Ӧ��D�������������Һ�壮ͨ������£�F��GΪ���壬��F��һ�������Դ��X��ĿǰӦ����㷺�Ľ�����y������ʳƷ������������ʼ��ת����ϵ��ͼ��ʾ�����������ȥ��
��ͼ��A��B�ɷ����кͷ�Ӧ��D�������������Һ�壮ͨ������£�F��GΪ���壬��F��һ�������Դ��X��ĿǰӦ����㷺�Ľ�����y������ʳƷ������������ʼ��ת����ϵ��ͼ��ʾ�����������ȥ��
 �ݱ�����ȫ��ÿ���˷ѵ�����ʳԼ��1000�ڶ֣����У�Լ300�ڶ��Ǽӹ�����ʳƷ����ͼ��һ����������ͼƬ��ͼ�е���������ʳƷ�������ʣ��ر��Ƕ��ӳ�������֬ʳƷ�ı�����������Ҫ���ã���ش��������⣺
�ݱ�����ȫ��ÿ���˷ѵ�����ʳԼ��1000�ڶ֣����У�Լ300�ڶ��Ǽӹ�����ʳƷ����ͼ��һ����������ͼƬ��ͼ�е���������ʳƷ�������ʣ��ر��Ƕ��ӳ�������֬ʳƷ�ı�����������Ҫ���ã���ش��������⣺