��Ŀ����
ij��һ�����ʵ����ǵ���ιۣ��ó���Ҫ��Ʒ֮һ��С�մ�̼�����ƣ����ι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����̼�����Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.3 g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ��ͼ��ʾ����������������⣺���������ðٷ�����ʾ��������С�����һλ���֣�

��1����Ʒ��̼�����Ƶ�����������
��2��ǡ����ȫ��Ӧʱ��������Һ�����ʵ�����������
��1��90.3% ��2��22.5%
��������̼�����������ᷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼������������ϵͼ��������25gϡ����ʱǡ����ȫ��Ӧ���ų����������̼������Ϊ4.4g��
��1���ɸ��ݻ�ѧ����ʽ���ɲ���������̼���������������Ʒ��̼�����Ƶ����������������Ʒ��̼�����Ƶ�����������
��2��ǡ����ȫ��Ӧʱ������ҺΪ�Ȼ�����Һ�����������Ȼ�����ԭ������е��Ȼ��ƺͷ�Ӧ���ɵ��Ȼ��������������ɣ���˼�����������ʱ��Ҫ�����ԭ���Ȼ��Ƽ����������Ȼ��Ƶ���������Һ���������������غ�����
�⣺����Ʒ��̼�����Ƶ�����Ϊx, �����Ȼ��Ƶ�����Ϊy
NaHCO3 + HClNaCl + CO2��+ H2O��1�֣�
84 58.5 44
x y 4.4 g
 x �� 8.4 g��1�֣�
x �� 8.4 g��1�֣�
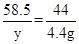 y �� 5.85 g��1�֣�
y �� 5.85 g��1�֣�
(1) ��Ʒ��̼�����Ƶ���������Ϊ�� 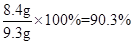 ��2�֣�
��2�֣�
(2) ǡ����ȫ��Ӧʱ��������Һ�����ʵ���������Ϊ��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�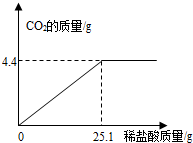 ij��һ�����ʵ����ǵ����Ƹۼ�ιۣ��ó���Ҫ��Ʒ֮һ��С�մ�̼�����ƣ����ι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����̼�����Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.3g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ��ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣�
ij��һ�����ʵ����ǵ����Ƹۼ�ιۣ��ó���Ҫ��Ʒ֮һ��С�մ�̼�����ƣ����ι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����̼�����Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.3g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ��ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣� ij��һ�����ʵ����ǵ���ιۣ��ó���Ҫ��Ʒ֮һ�Ǵ���ι۽�����ͬѧ�Ǵ���һЩ������������Ĵ�����Ʒ�����ⶨ����̼���Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ12g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ����ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣�
ij��һ�����ʵ����ǵ���ιۣ��ó���Ҫ��Ʒ֮һ�Ǵ���ι۽�����ͬѧ�Ǵ���һЩ������������Ĵ�����Ʒ�����ⶨ����̼���Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ12g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ����ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣� ij��һ�����ʵ����ǵ����Ƹۼ�ιۣ��ó���Ҫ��Ʒ֮һ��С�մι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����С�մ�������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.4g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ��ͼ��ʾ��NaHCO3+HCl�TNaCl+H2O+CO2����������������С�����һλ���֣�
ij��һ�����ʵ����ǵ����Ƹۼ�ιۣ��ó���Ҫ��Ʒ֮һ��С�մι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����С�մ�������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.4g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ��ͼ��ʾ��NaHCO3+HCl�TNaCl+H2O+CO2����������������С�����һλ���֣� ij��һ�����ʵ����ǵ����Ƹۼ�ιۣ��ó���Ҫ��Ʒ֮һ��С�մι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����С�մ�������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.4g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ��ͼ��ʾ��NaHCO3+HCl�TNaCl+H2O+CO2����������������С�����һλ���֣�
ij��һ�����ʵ����ǵ����Ƹۼ�ιۣ��ó���Ҫ��Ʒ֮һ��С�մι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����С�մ�������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.4g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ��ͼ��ʾ��NaHCO3+HCl�TNaCl+H2O+CO2����������������С�����һλ���֣�