��Ŀ����
����Ŀ���������ǻ�����������������֯����Ҫԭ�ϣ�����ͨ��ʳ���õĵ�������θ������ˮ��Ӧ�����ɰ����ᡣ�����ᣨ��ѧʽΪ![]() ���������е�һ�֡���ش��������⡣
���������е�һ�֡���ش��������⡣
��1���������и�Ԫ�ص�������C��H��O��N��S=_____��
��2���������е�Ԫ�ص���������Ϊ_____��
��3���ϸ��̷�ÿ100�˺�������Լ18�ˣ��������е�Ԫ�ص�ƽ����������Ϊ16%����ÿ100�˺ϸ��̷��е�Ԫ�ص�����Ϊ_____�ˣ��ֲⶨij�̷�ÿ100���к��е�Ԫ��0.5�ˣ��������̷������������̷�����_____�������ϸ����������ϸ������̷ۡ�
���𰸡�60��11��32��14��32 9.4% 2.88 ���ϸ�
��������
��1�����ݻ������и�Ԫ��������=��Ԫ�ص����ԭ��������ԭ�Ӹ���֮�ȣ��ɵõ����ᣨ��ѧʽΪ![]() ����̼���⡢��������������Ԫ�ص�������Ϊ��12��5������1��11������16��2������14��1������32��1��=60��11��32��14��32��
����̼���⡢��������������Ԫ�ص�������Ϊ��12��5������1��11������16��2������14��1������32��1��=60��11��32��14��32��
��2���������е�Ԫ�ص���������![]() ��
��
��3���������⣬�ϸ��̷�ÿ100���к�������Լ18�ˣ��������е�Ԫ�ص�ƽ����������Ϊ16%����ÿ100g�ϸ��̷��е�Ԫ�ص�����Ϊ18g��16%=2.88g��
��4�����Ϸ�����֪ÿ100g�ϸ��̷��е�Ԫ�ص�����Ϊ18g��16%=2.88g��0.5g���ʸ��̷۲��ϸ�

����Ŀ������ͬѧͨ���Ķ��������ϵ�֪��DZˮͧ�г��ù������ƣ�Na2O2����Ϊ���������йط�Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2CO2�T2Na2CO3+O2��2Na2O2+2H2O�T4NaOH+O2��������������ͼ����װ������ȡCO2����֤����Na2O2�ķ�Ӧ��
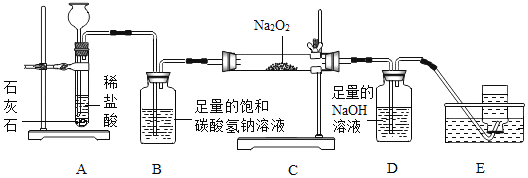
��1��װ��B��������_____��
��2����Ӧһ��ʱ���װ��E���ռ�����������Ҫ��_____����Ӧ��װ��CӲ�ʲ������й���ijɷ���ʲô������Ϊ���ֽ���������̽����
����������裩
����һ Na2CO3
�����Na2CO3��Na2O2
������Na2CO3��NaOH
������������_____
�����ʵ�飩
ʵ����� | ʵ������ | ʵ����� |
��ȡ������Ʒ���Թ��У�����������ˮ���� | ������ȫ�ܽ⣬_____ | ��Ʒ��һ��û��Na2O2 |
��ȡ����ʵ����������Һ����һ�Թ��У����������BaCl2��Һ���� | �а�ɫ�������� | ��������ȷ |
��ȡ����ʵ���������ϲ���Һ����һ�Թ��У�����_____��Һ���� | �а�ɫ�������� |
����˼�����ۣ���Ӧ��װ��CӲ�ʲ������еĹ��庬��NaOH��ԭ�������_____��
����Ŀ������-���-���š����ر����ǻ�ѧ���صı�ʾ���ʼ���仯��˼ά������
��1������������β�������������йػ�ѧ��Ӧ����ʾ��ͼ��ͼ����ʾ��Ӧ�Ļ�ѧ����ʽΪ___________________��

��2��ij��ѧ��ȤС���ͬѧ̽����ijЩ�ᡢ���֮���Ƿ������ֽⷴӦ��
���� | ���� | ʵ����� | ���� | |
ʵ��һ |
| ________ | �÷�Ӧ����ʵ�ʣ������ӷ��ű�ʾ�� :
| ���ֽⷴӦ��ʵ������Һ������֮�����Ӧ������__________ |
ʵ��� |
| ��Һ��ɫ�ɺ�ɫǡ�ñ�Ϊ��ɫ |
| |
ʵ���� |
| ������ɫ���� | �÷�Ӧ����ʵ�ʣ������ӷ��ű�ʾ�� : ________ |
[Ǩ��Ӧ��]����������ˮ���ܴ������棬���γ���ɫ��Һ����_______________������ĸ��
a. Fe2+Na+NO3-Cl-
b. K+Na+OH- SO42-
c.H+K+SO42-OH-
d. Ba2+NO3-CO32-Cl-
����Ŀ����ѧ��ѧ������ѧ���úû�ѧ��ѧϰС��Ϊ̽����������ͭ�Ļ��ǿ������չ�����»��
���������ϣ��������ڳ�������������е�������Ӧ�������ܵ���������Ĥ��
���Ա�ʵ�飩
��� | ���� | ���� |
�� | ������δ��ĥ����˿����CuSO4��Һ�� | ���������� |
�� | �������ĥ�����˿����CuSO4��Һ�� | ��˿����������ɫ���� |
�� | ������δ��ĥ����˿����CuCl2��Һ�� | ��˿����������ɫ���� |
��1���������ڳ�������������е�������Ӧ�������ܵ���������Ĥ����д����ط�Ӧ����ʽ_____�Ƚ�ʵ���Һ�ʵ��_____����ס��������ɵ�֪����ĥ���ƻ���������Ĥ��
��2��ʵ�����з�Ӧ�Ļ�ѧ����ʽΪ_____���ݴ˿�֪�������Al��Cu_____���ǿ������������
��3��С��ͬѧ��ʵ�����������з�������ΪH2O����������Ĥ���ƻ����á����˹۵����ϱ�����ͬѧ����������_____��
���²���̽����
С��ͬѧ���ʵ������������ۺ�²⣺Cl���ƻ�����������Ĥ��
Ϊ����˲²��Ƿ���ȷ��������������֧�Թ��м�����ͬ��CuSO4��Һ�������������δ��ĥ����˿��Ȼ��������µ�̽����
���� | ���� | ���� | ���� |
��1����һ֧�Թ����ټ���NaCl���� | ��˿����������ɫ���� | ��������Ĥ���ƻ� | Na+��_____���ƻ���������Ĥ |
��2������һ֧�Թ����ټ���Na2SO4���� | _____ | ��������Ĥδ���ƻ� | Na+��SO42���������ƻ���������Ĥ |
�������뷴˼��
�ó����ۣ�ǰ���²�_____�����ȷ������ȷ������
�ܽᷴ˼������̽����������˱ȽϷ��Ϳ��Ʊ�������







