��Ŀ����
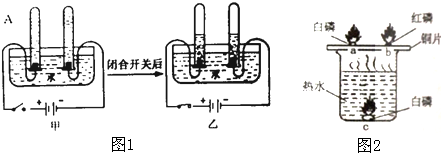
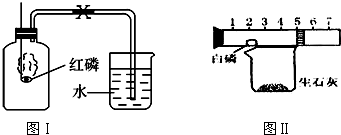
�����г���̼��ơ����������������ΪĦ������ijͬѧ������ͼ��ʾ��װ�ö�����Ħ������̼��Ƶĺ�������̽����
ʵ��ԭ����
�ⶨCװ��������BaCO3������������ͨ������ȷ��Ħ������CaCO3������������
�������ϣ�
CO2+Ba��OH��2�TBaCO3��+H2O�������������ɷ���������ʱ�����������
����ݸ�ͬѧ��̽�����̻ش��������⣺

��1��ʵ�����ʱ��Ҫ��������ͨ�������Ŀ���ǣ� ��Dװ�õ������� ��
��2����û��Aװ�ã�ֱ��ͨ�����������CaCO3���������� ���ƫ����ƫС�����䡱����
��3��ʵ����ȷ��ȡ����������Ʒ��ÿ��8g���������βⶨ�����BaCO3��ƽ������Ϊ3.94g������3.94g BaCO3��Ҫ0.88g������̼����Ħ������̼��Ƶ�������������д��������̣�
ʵ��ԭ����
�ⶨCװ��������BaCO3������������ͨ������ȷ��Ħ������CaCO3������������
�������ϣ�
CO2+Ba��OH��2�TBaCO3��+H2O�������������ɷ���������ʱ�����������
����ݸ�ͬѧ��̽�����̻ش��������⣺

��1��ʵ�����ʱ��Ҫ��������ͨ�������Ŀ���ǣ�
��2����û��Aװ�ã�ֱ��ͨ�����������CaCO3����������
��3��ʵ����ȷ��ȡ����������Ʒ��ÿ��8g���������βⶨ�����BaCO3��ƽ������Ϊ3.94g������3.94g BaCO3��Ҫ0.88g������̼����Ħ������̼��Ƶ�������������д��������̣�
���㣺ʵ��̽�����ʵ���ɳɷ��Լ�����,��������ļ�������ӷ���,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺��ѧ̽��
��������1��ʵ�����ʱ��������ͨ�����ʱ���ܹ��ѷ�Ӧ�����Ķ�����̼��������������Һ���գ�ʹʵ������ȷ��
Dװ���еļ�ʯ���ܹ�����ˮ�Ͷ�����̼���Ӷ���ֹ�����еĶ�����̼��������������Һ�У�Ӱ��ʵ������
��2����û��Aװ�ã�ֱ��ͨ���������ô�����еĶ�����̼�ᱻ����������Һ���գ�Ӱ��ʵ������
��3�����ݶ�����̼���������Լ���̼��Ƶ���������һ�����Լ���Ħ������̼��Ƶ�����������
Dװ���еļ�ʯ���ܹ�����ˮ�Ͷ�����̼���Ӷ���ֹ�����еĶ�����̼��������������Һ�У�Ӱ��ʵ������
��2����û��Aװ�ã�ֱ��ͨ���������ô�����еĶ�����̼�ᱻ����������Һ���գ�Ӱ��ʵ������
��3�����ݶ�����̼���������Լ���̼��Ƶ���������һ�����Լ���Ħ������̼��Ƶ�����������
����⣺��1��ʵ�����ʱ��Ҫ��������ͨ�������Ŀ���ǣ�ʹ��Ӧ���ɵĶ�����̼ȫ��������������Һ���գ�Dװ�õ������ǣ���ֹ�����еĶ�����̼������������Һ���գ�
���ʹ��Ӧ���ɵĶ�����̼ȫ��������������Һ���գ���ֹ�����еĶ�����̼������������Һ���գ�
��2����û��Aװ�ã�ֱ��ͨ���������ô�����еĶ�����̼�ᱻ����������Һ���գ��ᵼ�²ⶨ��̼�������ƫ�Ӷ����²��CaCO3����������ƫ��
���ƫ��
��3���⣺��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2����
100 44
x 0.88g
=
��
x=2g��
Ħ������̼��Ƶ���������Ϊ��
��100%=25%��
��Ħ������̼��Ƶ���������Ϊ25%��
���ʹ��Ӧ���ɵĶ�����̼ȫ��������������Һ���գ���ֹ�����еĶ�����̼������������Һ���գ�
��2����û��Aװ�ã�ֱ��ͨ���������ô�����еĶ�����̼�ᱻ����������Һ���գ��ᵼ�²ⶨ��̼�������ƫ�Ӷ����²��CaCO3����������ƫ��
���ƫ��
��3���⣺��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2����
100 44
x 0.88g
| 100 |
| 44 |
| x |
| 0.88g |
x=2g��
Ħ������̼��Ƶ���������Ϊ��
| 2g |
| 8g |
��Ħ������̼��Ƶ���������Ϊ25%��
������ʵ���ǻ�ѧ����Ҫ��ɲ��֣���ȷ��ʵ������ǵó���ѧ���۵�ǰ������֮һ�����Ҫѧ�����ʵ�顢����ʵ�顢����ʵ�飬Ϊ�ó���ȷ�Ľ��۵춨������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���з�����ֻ�������壬û�к��������ǣ�������
| A��2N |
| B��N2 |
| C��K |
| D��H2O |
 ��ͼ��ʾ��װ�ò��������������ĺ�������д���пհף�
��ͼ��ʾ��װ�ò��������������ĺ�������д���пհף�