��Ŀ����
Ϊ�˲ⶨ���������Ȼ��Ƶ�̼�����ƣ�NaHCO3�����������������ijͬѧ���øù���������ϡ���ᷴӦ������������ʵ�飬������ص�ʵ�����ݼ�¼���±���
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�������������/g | 10 | 10 | 15 |
| ����ϡ���������/g | 60 | 70 | 50 |
| ����CO2������/g | 3.3 | 3.3 | 3.3 |
��2��ϡ�������ʵ�����������________��
��3�����ϱ�����������ȡ��������������ϡ�����������Ϊ________ʱ�������ù��������е�NaHCO3��ϡ����ǡ����ȫ��Ӧ��
��4��ȡ10g�ù���������ϡ����ǡ����ȫ��Ӧ����������Һ�����ʵ�������������������ȷ��0.001������%��ʾ����
NaHCO3+HCl�TNaCl+H2O+CO2��
84 36.5 58.5 44
X z Y 3.3g
 x=6.3g
x=6.3g y=4.3875g
y=4.3875g =
= z=2.7375g
z=2.7375g�������NaHCO3������������
 ��100%=63%
��100%=63%��2��ϡ�������ʵ����������ǣ�
 ��100%=5.48%
��100%=5.48%��3�����ϱ�����������ȡ��������������ϡ�����������Ϊ1��5 ʱ�������ù��������е�NaHCO3��ϡ����ǡ����ȫ��Ӧ��
��4��������Һ�����ʵ���������Ϊ��
 ��100%=14.25%
��100%=14.25%�ʴ�Ϊ����1��63%����2��5.48%����3��1��5����4��������Һ�����ʵ���������14.25%��
��������һ���������ݿ�֪10g̼��������Ʒ��ϡ������ȫ��Ӧ����3.3g������̼�������ɶ�����̼����������̼��������ϡ���ᷴӦ�Ļ�ѧ����ʽ���Լ�����������NaHCO3��������������һ���������ݿ�֪50gϡ������ȫ��Ӧ����3.3g������̼�����10gʯ��ʯ��50gϡ����ǡ����ȫ��Ӧ������������ڶ���Ĵ𰸣��������ϼ�������10g�ù���������ϡ����ǡ����ȫ��Ӧ�����ɵ��Ȼ��Ƶ���������Һ�е��Ȼ���Ӧ�������ɵ��Ȼ��ƺ�ԭ������л��е��Ȼ���֮�ͣ��ٸ�����Һ�����ʵ�����������ʽ���м��㣮
���������⿼����Ǹ��ݻ�ѧ����ʽ���йؼ��㣬ѧ�����ÿ��Ʊ���������ȷ�ķ����������е������ǽ�����Ĺؼ���

 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�ijУ��ѧ��ȤС��ι��Ƽ���������Ϣ���������������о���
[��������]
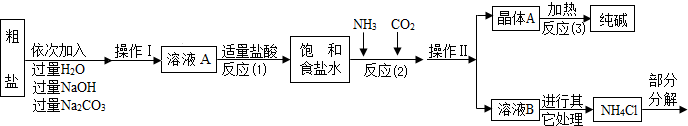
������ԭ�ϴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
������ԭ����Ӧ��2����NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl������þ���A����ʹ�������ȣ��ɷֽ��Ƶô�����ֳ����������
���Ȼ�立ֽ�Ļ�ѧ����ʽ��NH4Cl NH3��+HCl����
NH3��+HCl����
�ܲ�������������ͼ��ʾ��
[��������]
��1������ҺA�е�������NaCl��______��______���ڲ����������Ϊ______��
��������NaOH��Һ�������dz�ȥ�����е�______��
��д������Na2CO3��Һ��������Ӧ�Ļ�ѧ����ʽ______��
��2���������������п�ѭ��ʹ�õ���______������ţ���
A��CO2��������B��NH3��������C��HCl�������� D��NaOH
[���̽��һ]��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ______��
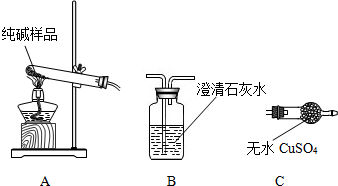
�����ʵ����鴿����Ʒ���Ƿ���о���A��������±���
| ѡ���װ�� | ʵ������ | ʵ����� |
| ______ | ______ | ��Ʒ��������A |

[���̽����]��4��ȡ������Ʒ��ˮ�ܽ⣬�����Һ�м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ______���ɴ�ȷ��������Ʒ��������NaCl��

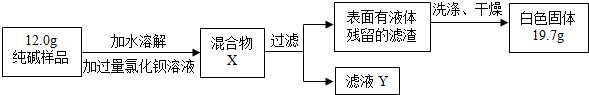
[���̽����]��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺
���жϼ����Ȼ�����Һ�Ƿ�����ĺ��ʷ�����______��Ȼ��۲������жϣ�
A�����û����X�����ϲ���Һ���ٵ������Ȼ�����Һ��B������ҺY�еμ������Ȼ�����Һ
���ж������Ƿ�ϴ�Ӹɾ������Բ�ȡ������ϴ��Һ�еμ�______��Ȼ��۲������жϣ�
A���Ȼ�����Һ������ B��ϡ���ᡡ����C��̼������Һ����D��ϡ����
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ______��д��������̣�4�֣�
[Mr��BaCl2��=208��Mr��Na2CO3��=106��Mr��BaCO3��=197Mr��NaCl��=58.5]��
ijУ��ѧ��ȤС��ι��Ƽ���������Ϣ���������������о���
���������ϡ�
������ԭ�ϴ����к����������������ʣ�MgCl2��CaCl2�������������ʡ�
������ԭ����Ӧ�ƣ�NaCl+ NH3 + CO2 + H2O= NaHCO3��+ NH4Cl������þ���A����ʹ�������ȣ��ɷֽ��Ƶô�����ֳ����������
���Ȼ�立ֽ�Ļ�ѧ����ʽ��NH4Cl �� NH3��+HCl����
�ܲ���������������ͼ��ʾ��
 |
���������ۡ�
��1������ҺA�е�������NaCl�� �� ���ڲ����������Ϊ ��
��������NaOH��Һ�������dz�ȥ�����е� ��
��д������Na2CO3��Һ��������Ӧ�Ļ�ѧ����ʽ ��
��2���������������п�ѭ��ʹ�õ��� ������ţ���
A��CO2 B��NH3 C��HCl D��NaOH
�����̽��һ����3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ ��
�����ʵ����鴿����Ʒ���Ƿ���о���A��������±���
�����̽��������4��ȡ������Ʒ��ˮ�ܽ⣬�����Һ�м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ ���ɴ�ȷ��������Ʒ��������NaCl��

| ѡ���װ�� | ʵ������ | ʵ����� |
| ��Ʒ��������A |
�����̽��������5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺
���жϼ����Ȼ�����Һ�Ƿ�����ĺ��ʷ����� ��Ȼ��۲������жϡ�
A.���û����X�����ϲ���Һ���ٵ������Ȼ�����Һ B.����ҺY�еμ������Ȼ�����Һ
���ж������Ƿ�ϴ�Ӹɾ������Բ�ȡ������ϴ��Һ�еμ� ��Ȼ��۲������жϡ�
A.�Ȼ�����Һ B.ϡ���� C.̼������Һ D.ϡ����
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ ��д��������̡�4�֣�
[Mr(BaCl2)=208 Mr(Na2CO3)=106 Mr(BaCO3)=197 ��Mr(NaCl)=58.5]
[��������]
������ԭ�ϴ����к����������������ʣ�MgCl2��CaCl2�������������ʣ�
������ԭ����Ӧ��2����NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl������þ���A����ʹ�������ȣ��ɷֽ��Ƶô�����ֳ����������
���Ȼ�立ֽ�Ļ�ѧ����ʽ��NH4Cl
 NH3��+HCl����
NH3��+HCl�����ܲ�������������ͼ��ʾ��

[��������]
��1������ҺA�е�������NaCl�� �� ���ڲ����������Ϊ ��
��������NaOH��Һ�������dz�ȥ�����е� ��
��д������Na2CO3��Һ��������Ӧ�Ļ�ѧ����ʽ ��
��2���������������п�ѭ��ʹ�õ��� ������ţ���
A��CO2 B��NH3 C��HCl D��NaOH
[���̽��һ]��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ ��
�����ʵ����鴿����Ʒ���Ƿ���о���A��������±���
| ѡ���װ�� | ʵ������ | ʵ����� |
| ��Ʒ��������A |

[���̽����]��4��ȡ������Ʒ��ˮ�ܽ⣬�����Һ�м������ϡHNO3���ٵμ�AgNO3��Һ���а�ɫ���������������ķ���ʽΪ ���ɴ�ȷ��������Ʒ��������NaCl��

[���̽����]��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺
���жϼ����Ȼ�����Һ�Ƿ�����ĺ��ʷ����� ��Ȼ��۲������жϣ�
A�����û����X�����ϲ���Һ���ٵ������Ȼ�����Һ B������ҺY�еμ������Ȼ�����Һ
���ж������Ƿ�ϴ�Ӹɾ������Բ�ȡ������ϴ��Һ�еμ� ��Ȼ��۲������жϣ�
A���Ȼ�����Һ B��ϡ���� C��̼������Һ D��ϡ����
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ ��д��������̣�4�֣�
[Mr��BaCl2��=208 Mr��Na2CO3��=106 Mr��BaCO3��=197Mr��NaCl��=58.5]��