��Ŀ����
 ��1��20��ʱ������֧�Թ��м���������ļס������ֹ������ʣ��ֱ����10gˮ��ʹ�����ܽ⣬�۲쵽��ͼ1��ʾ������20��ʱ��
��1��20��ʱ������֧�Թ��м���������ļס������ֹ������ʣ��ֱ����10gˮ��ʹ�����ܽ⣬�۲쵽��ͼ1��ʾ������20��ʱ����2����ͼ2�б�ʾ�����ʵ��ܽ�����ߵ���
��3����10��a�ı�����Һ���µ�20�棬��������������
��4��Ҫ�Ӻ��������ҵļ��������ᴿ�ף��ɲ��õķ�����
���㣺������Һ�Ͳ�������Һ,�ᾧ��ԭ������������Ӧ��,������Һ�Ͳ�������Һ�ת��ķ���,�����ܽ������������
ר�⣺��Һ����Һ���ܽ��
��������1�����ݱ�����Һ�ĺ��������Һ�ı��͡������ͣ�
��2������20��ʱ�ܽ�ȴ�С�����ס��ҵ��ܽ�����ߣ����ݼ����ʵ��ܽ�����¶ȵı仯�������Ҫʹ�Թ���ʣ��ļ�������ܽ�ɲ��õķ�����
��3��������Һ����ɷ����������������ı仯��
��2������20��ʱ�ܽ�ȴ�С�����ס��ҵ��ܽ�����ߣ����ݼ����ʵ��ܽ�����¶ȵı仯�������Ҫʹ�Թ���ʣ��ļ�������ܽ�ɲ��õķ�����
��3��������Һ����ɷ����������������ı仯��
����⣺��1����ͼʾ1��֪�������ʵ���Һ�������ܽ�������ˣ������DZ�����Һ��
��2����ͼ2��ʾ��20��ʱ���ܽ������bλ���ܽ������a�·���������ʱa���ܽ�ȴ���b��ͼ1��ʾ������ȫ���ܽ�����ʼ��й�������ʣ�࣬˵����ʱ�����ʵ��ܽ�ȴ��ڼ����ʣ���ˣ��ɵ�ͼ2��b��ʾ�����ʵ��ܽ�����ߣ������ʵ��ܽ�����¶ȵ����߶�����Ҫʹ�Թ���ʣ��ļ�������ܽ�ɲ��õķ����������¶Ȼ��ˮ��
��3����10��ʱa�ı�����Һ���µ�20�棨�ܼ�������������Һ����ɲ��䣬���ԣ������������������䣻
��4��Ҫ�Ӻ��������ҵļ��������ᴿ�ף��ɲ��õķ����������ᾧ��
�ʴ�Ϊ����1���ף���2��b�����»��ˮ����3�����䣻��4�������ᾧ��
��2����ͼ2��ʾ��20��ʱ���ܽ������bλ���ܽ������a�·���������ʱa���ܽ�ȴ���b��ͼ1��ʾ������ȫ���ܽ�����ʼ��й�������ʣ�࣬˵����ʱ�����ʵ��ܽ�ȴ��ڼ����ʣ���ˣ��ɵ�ͼ2��b��ʾ�����ʵ��ܽ�����ߣ������ʵ��ܽ�����¶ȵ����߶�����Ҫʹ�Թ���ʣ��ļ�������ܽ�ɲ��õķ����������¶Ȼ��ˮ��
��3����10��ʱa�ı�����Һ���µ�20�棨�ܼ�������������Һ����ɲ��䣬���ԣ������������������䣻
��4��Ҫ�Ӻ��������ҵļ��������ᴿ�ף��ɲ��õķ����������ᾧ��
�ʴ�Ϊ����1���ף���2��b�����»��ˮ����3�����䣻��4�������ᾧ��
�������������ʼ���ˮ�г���ܽ����Һ������δ�ܽ�Ĺ������ʵ���Һһ��Ϊ���¶��µı�����Һ��

��ϰ��ϵ�д�
�����Ŀ
��ѧ�����������������أ�������ijͬѧ�����߷�ʽ��ijһ����֪ʶ���й��ɵ���������в���ȷ���ǣ�������
| A����ѧ����ϣ��������µIJ���-���������� ������Դ�����IJ���-�����ȹ��� |
| B����ѧ��������ũ����Ҷɫ����-ʩ�õ��� �����-���Ϸ��� |
| C����ѧ�뽡��������ȱ�ٷ�Ԫ��-��ȣ�� ��������-��ʳ��ʹ���ж����� |
| D����ѧ����Դ���ƾ�-��ʯȼ�� ����-��ʯȼ�� |
������ijͬѧ���Ļ�ѧС�ʼǣ�������ȷ���ǣ�������
| A�����������벻��Ӫ���أ��߲�ˮ��Ϊ�����ṩ����ҪӪ�����ǵ����� |
| B��ʯī�ͽ��ʯ����̼�ĵ��ʣ����ʯīת��Ϊ���ʯ���������仯 |
| C��ʳƷ�ı�������������ֱ�ӵĹ�ϵ |
| D��ú��ʯ�͡����鶼�ǻ�ʯȼ�ϣ����͡�ú�Ͷ���ʯ�ͼӹ���Ʒ |
���г��ӷ����ͻ�ѧ����ʽ��д����ȷ���ǣ�������
A��CaO�л���CaCO3������ȼ�գ�CaCO3
| ||||
B��CO2�л���CO����ȼ��2CO+O2
| ||||
| C��������NaOH��Һ��ȥCO2 �����л�������HCl��2NaOH+CO2=Na2CO3+H2O | ||||
| D��FeCl2�л���CuCl2������п�ۣ�Zn+CuCl2=ZnCl2+Cu |

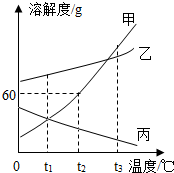 ��ͼΪ�ס��ҡ����������ʵ��ܽ�����ߣ���ͼ�ش�
��ͼΪ�ס��ҡ����������ʵ��ܽ�����ߣ���ͼ�ش�